Referring Patients Into Clinical Trials
Learn more about clinical trials and find a trial that might be right for you. Clinical trials are voluntary research studies conducted in people and designed to answer specific questions about.

Learn About Clinical Studies ClinicalTrials.gov Study, Learning, Clinic
Learn about clinical trials for people with cancer. AIDS Clinical Trials and Information Services (ACTIS) or call 1-800-TRIALS-A (1-800-874-2572). Locate clinical trials for people.
Celebrating 20 Years of ClinicalTrials.gov and Looking to the Future
Format. Linking PubMed and ClinicalTrials.gov (NCBI Minute) ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies. Citations in PubMed that report clinical trial results have links to the original studies in the ClinicalTrials database. You will learn two ways to filter PubMed searches for.
How to Understand and Get the Most Out of ClinicalTrials.gov CLL Society
(Funded by Novo Nordisk; STEP 1 ClinicalTrials.gov number, NCT03548935). Once-Weekly Semaglutide in Adults with Overweight or Obesity N Engl J Med. 2021 Mar 18;384(11):989-1002. doi: 10.1056/NEJMoa2032183. Epub 2021 Feb 10. Authors John P H Wilding 1. STEP 1 Study Group:

About ClinicalTrials.gov Clinical research, Medical research, Infographic
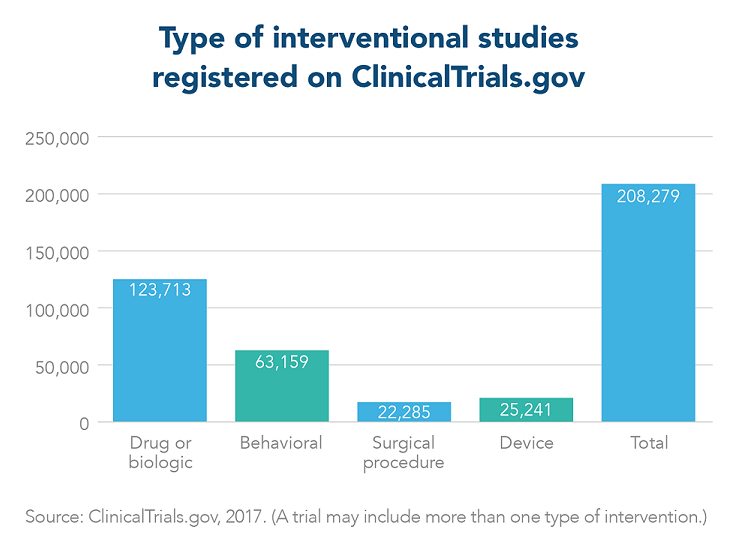
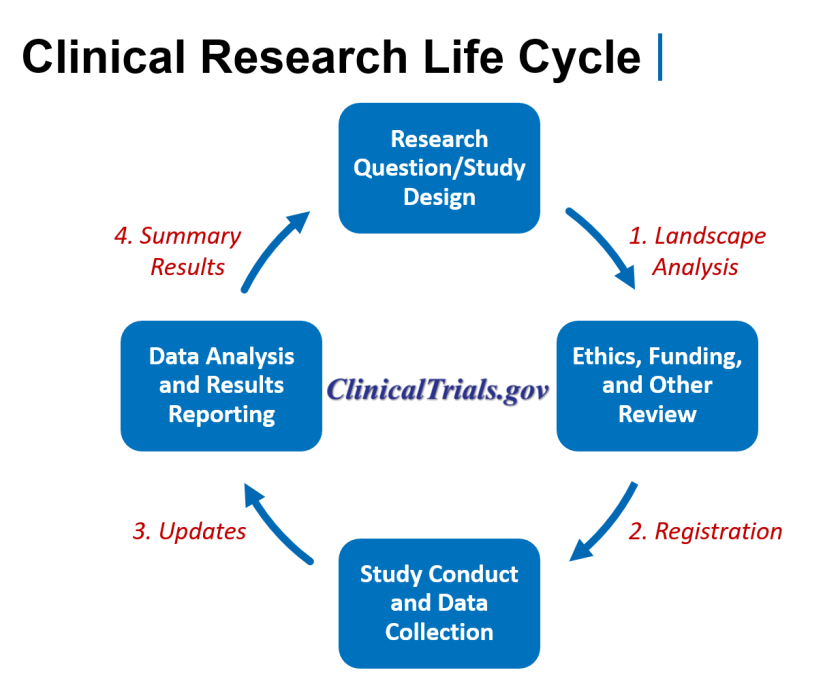
The ClinicalTrials.gov website was launched in 2000 by the NIH National Library of Medicine and is the largest clinical trial registry worldwide. The purpose of this analysis is to describe the composition and methodologic features of clinical trials as registered on ClinicalTrials.gov and to identify trends over time.

Completed Clinical Trials Results From Mesothelioma Studies
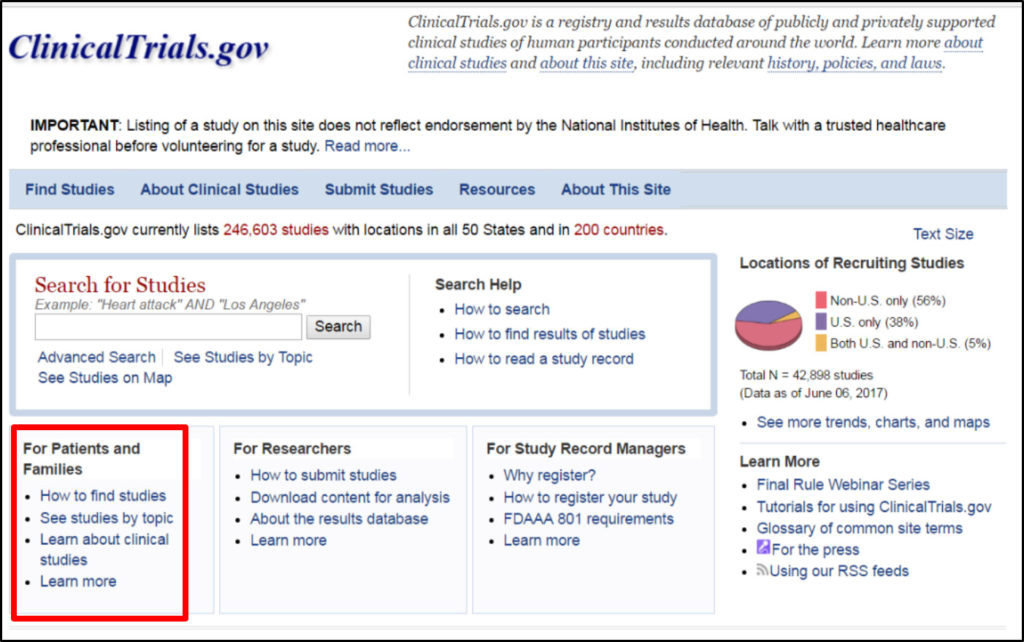
More than 4 million visitors use the website monthly to find and learn about clinical studies. Visit the modernized ClinicalTrials.gov website and discover the newest features for yourself! To allow users to adapt to the modernized site, the former ClinicalTrials.gov website will remain available under https://classic.clinicaltrials.gov/ until.

SciCrunch Research Resource Resolver
The notion of including women in clinical trials used to be revolutionary — which means many diagnostics and treatments were developed without women in mind and thus failed to account for women.

Navigating ClinicalTrials.gov YouTube
Clinical trials are research studies that test how well new medical approaches work in people. Each study answers scientific questions and tries to find better ways to prevent, screen for, diagnose, or treat a disease. Clinical trials may also compare a new treatment to a treatment that is already available. Every clinical trial has a protocol.

The Pitfalls of ClinicalTrials.gov
Abstract. We reviewed foundational concepts in artificial intelligence (AI) and machine learning (ML) and discussed ways in which these methodologies may be employed to enhance progress in clinical trials and research, with particular attention to applications in the design, conduct, and interpretation of clinical trials for neurologic diseases.

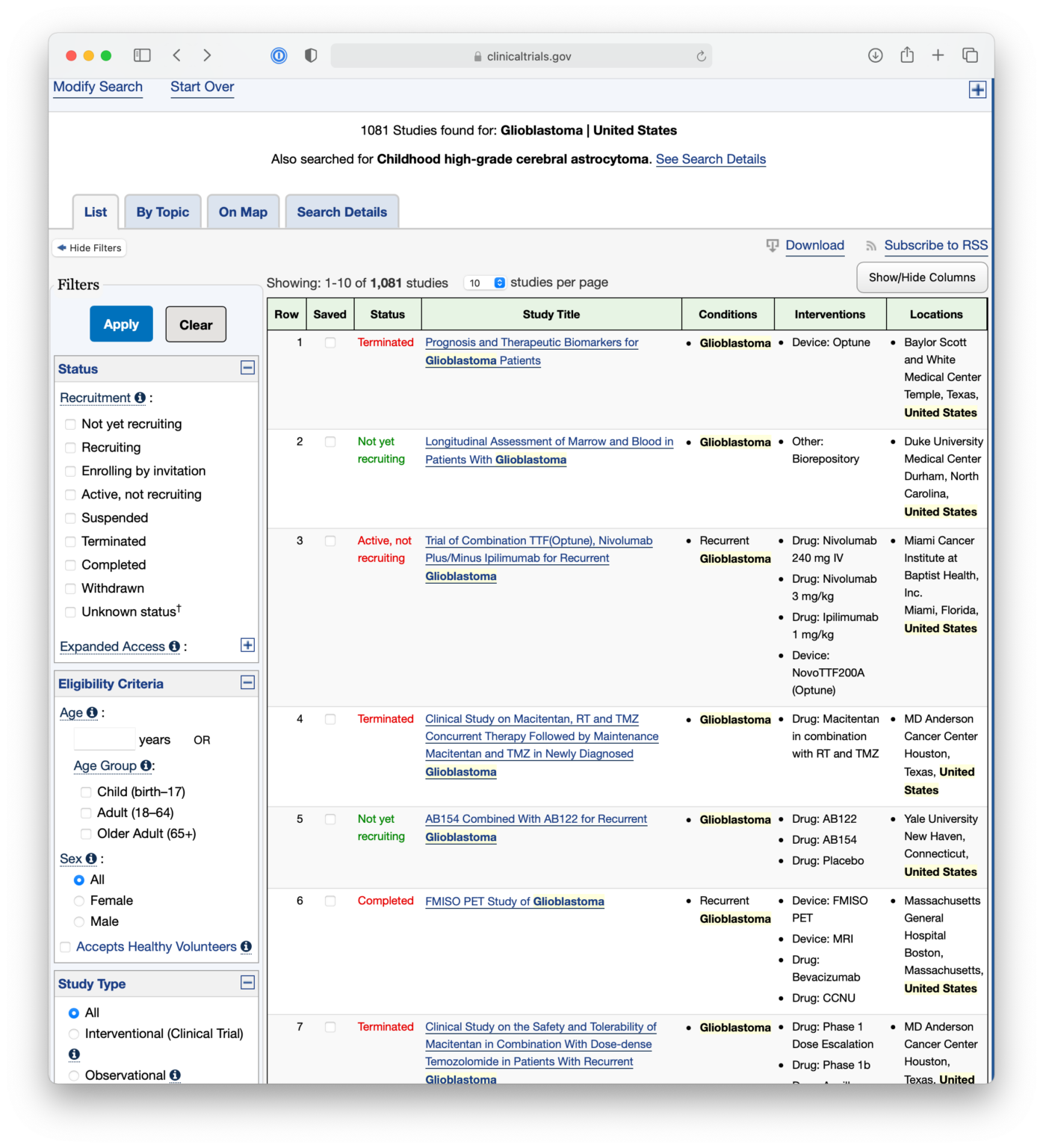
Finding Trials on ClinicalTrials.gov National Institutes of Health (NIH)
What are Clinical Trials? Clinical trials, part of clinical research, look at new ways to prevent, detect, or treat disease. They can lead to medical advances that help improve people's health and quality of life. Many people don't participate in clinical trials for various reasons—fear, lack of trust, or lack of awareness, to name just a.

2 Clinicaltrials.gov register your study YouTube
Two phase-2 clinical trials have begun to test the safety and effectiveness of three treatments for adults with autonomic nervous system dysfunction from Long Covid.. The current list of sites for the trials can be found on ClinicalTrials.gov; additional sites will be added as they begin enrolling participants.. To learn more about.
How To Find Clinical Trials Using ClinicalTrials.gov
The ClinicalTrials.gov API version 2.0 is now available on the modernized ClinicalTrials.gov website. The API, or application programming interface, is a tool to help researchers and developers access the data in ClinicalTrials.gov study records. This new version differs from the legacy API on the classic ClinicalTrials.gov website in important.

Registering and Reporting Results to ClinicalTrials.gov YouTube
Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read the disclaimer on ClinicalTrials.gov for details. Before participating in a study, talk to your health care provider and learn about the risks and potential benefits. At the NIH Clinical Center in Bethesda, Maryland

Importing Records into Papers from ClinicalTrials.gov Papers Support
What researchers learned (the study results) New updates will make it easier to find and learn about the studies that are relevant to you and the people in your life. The updated ClinicalTrials.gov website will include a new, more modern look and user-friendly features, like: Improved search and new filters to help you find the right information.

Clinicaltrials Gov Usa
A clinical study involves research using human volunteers (also called participants) that is intended to add to medical knowledge. There are two main types of clinical studies: clinical trials (also called interventional studies) and observational studies. ClinicalTrials.gov includes both interventional and observational studies.

ClinicalTrials.gov Tutorial YouTube
Learn about the possible risks and benefits of joining a clinical trial and questions to ask about trials. Explains clinical trials, including what they are, why they are important, things to think about when deciding to take part, and questions to ask your doctor.