
How to Calculate the Formal Charges for PO4 3 (Phosphate ion) YouTube
PO4 3- Lewis structure. In the lewis structure of PO43-, phosphorus is the central atom as it is the least electronegative. And all the four oxygen atoms are kept surrounding it. Total electron pairs used to form bonds are determined by dividing the number of total valence electrons by two. For, PO43- ion, the total pairs of electrons are 16.

Lewis Dot Structure of Phosphate (PO4 3).....No More Confusion
Key Takeaways The PO4 3- ion has a Lewis structure with a central phosphorus atom bonded to four oxygen atoms. The phosphorus atom has a formal charge of +3, while each oxygen atom has a formal charge of -1. The Lewis structure of PO4 3- shows that it has a tetrahedral molecular geometry.
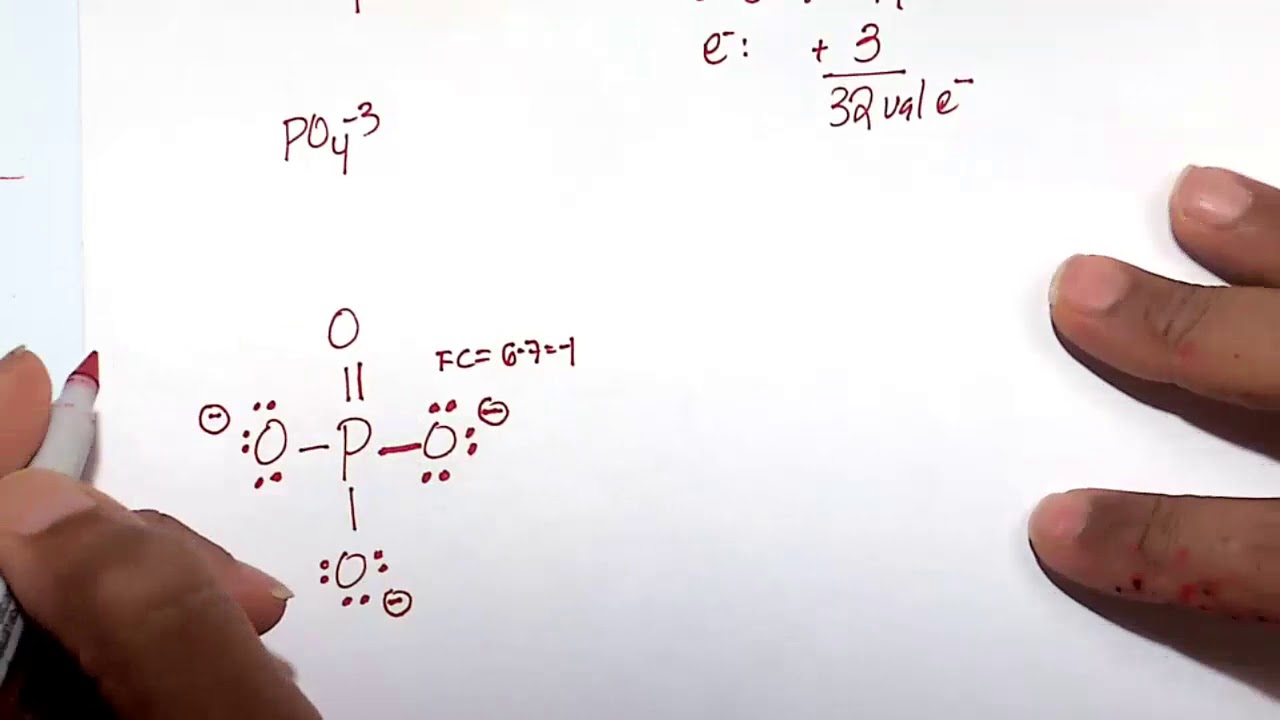
Resonance The resonance structure of the phosphate ion (PO4(3)) YouTube
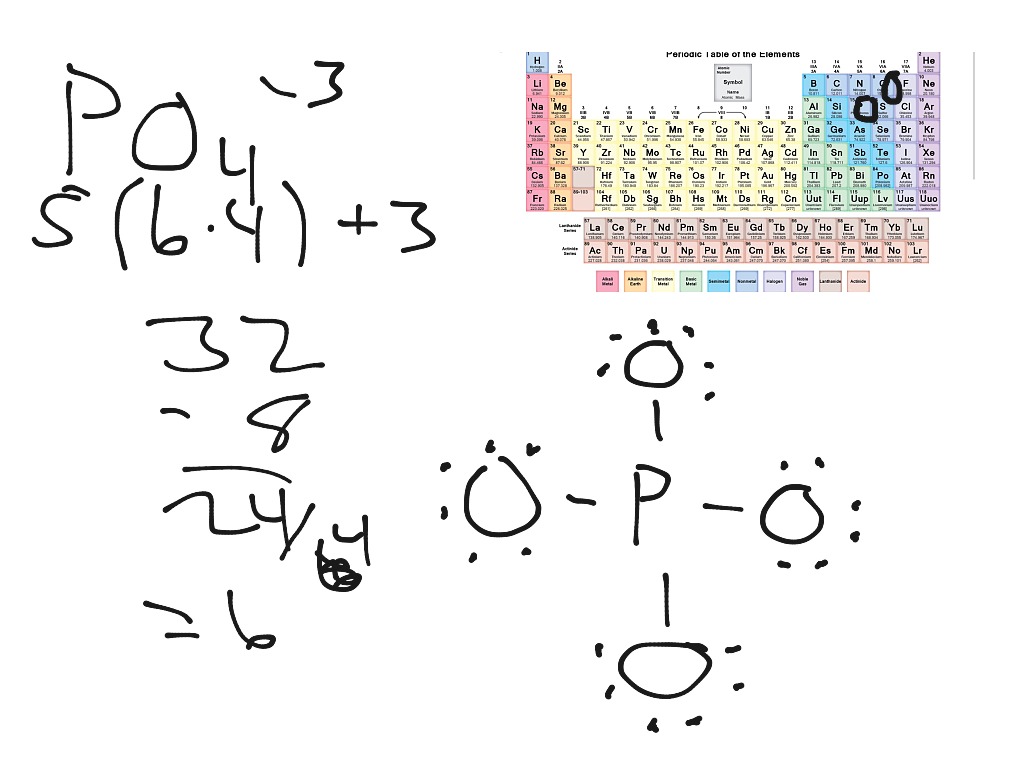
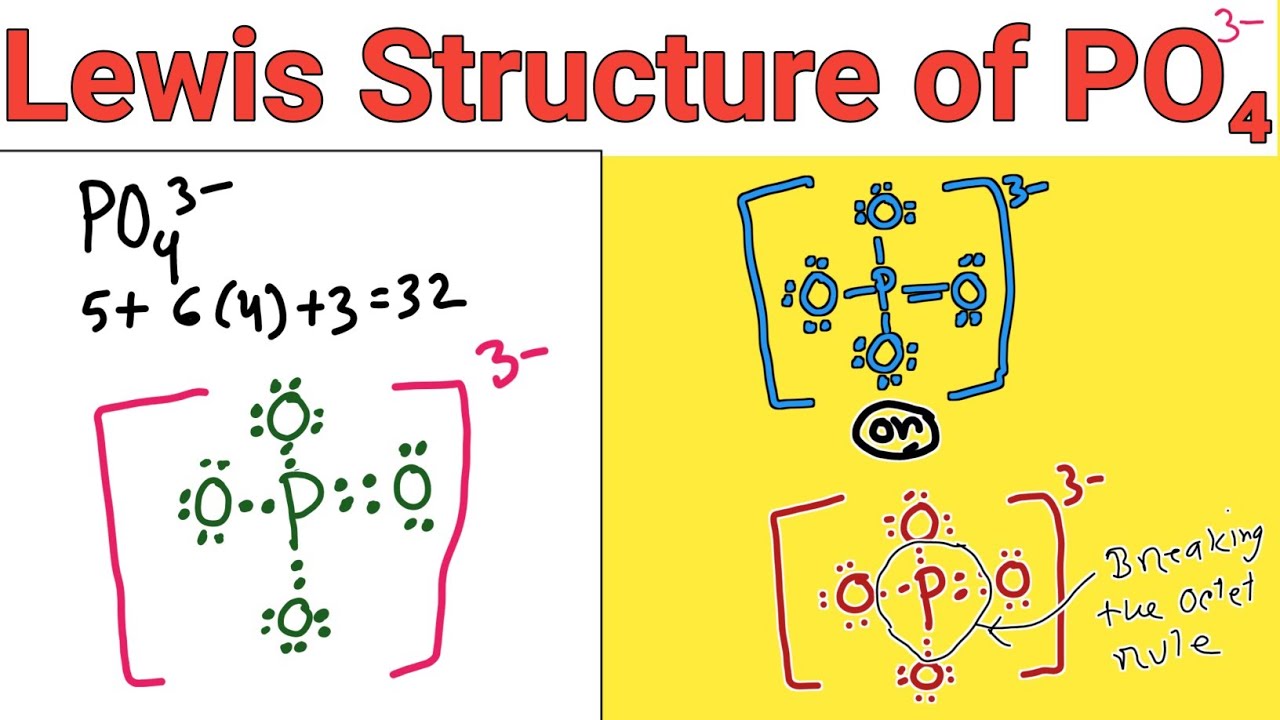
About this tutor ›. If we start at the beginning by drawing the Lewis dot structure we start with valence electrons. P has 5 ve. each O has 6 ve so that's a total of 4x6 = 24 ve. We add 3 more for the 3- charge for a total of 32 valence electrons. So, this can be written with P at the center and 4 O atoms attached with single bonds and all O.
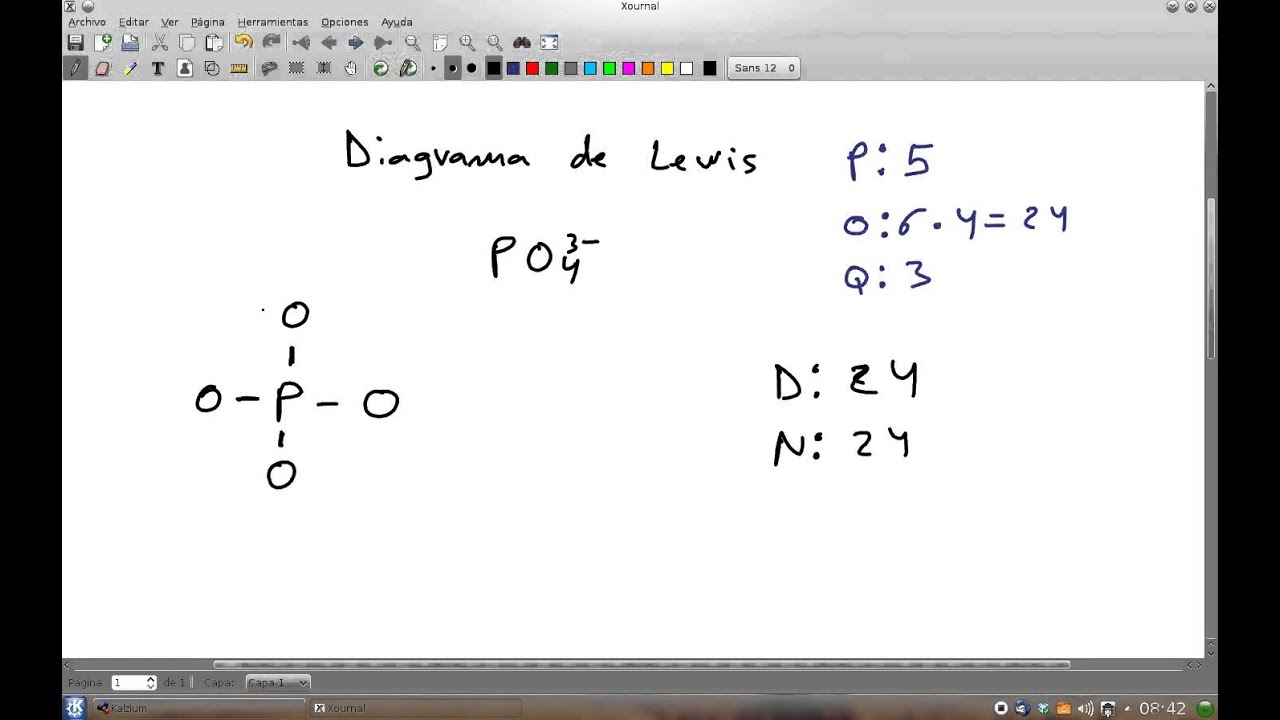
Diagrama de Lewis del ion fosfato PO4(2) YouTube
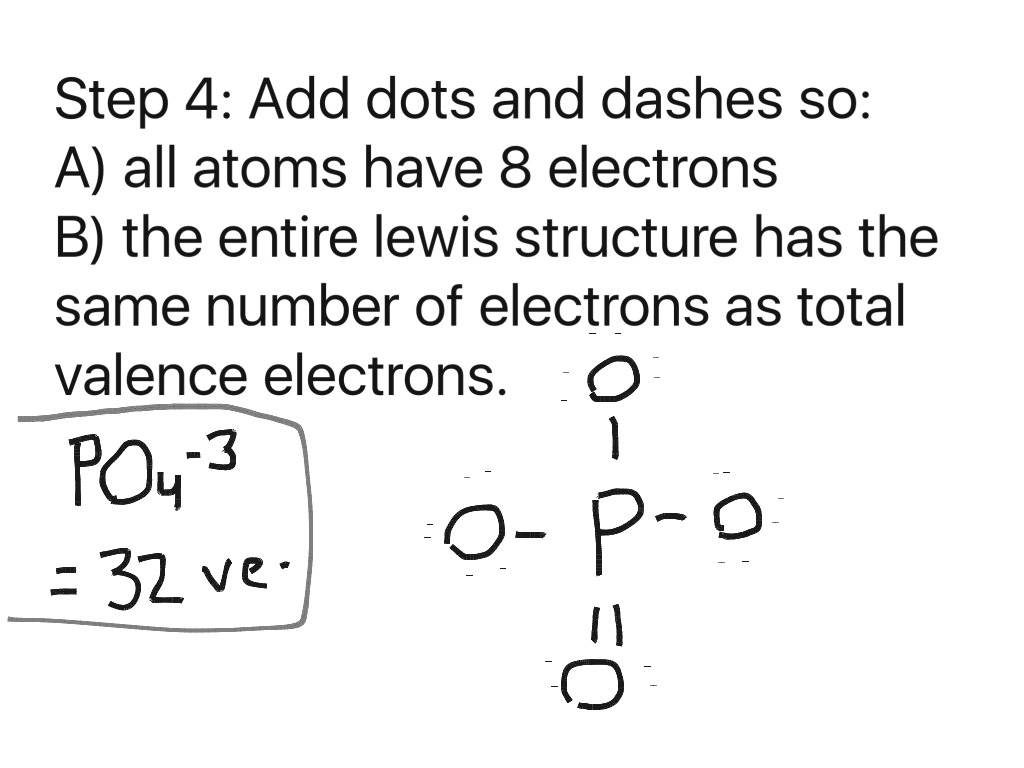
Step 4 in determining the Lewis Structure of PO4(3-) PO 4 3-Step 4 No Change from step 3 as we have no electrons left over. Picture so Far: Total Valence Electrons: 32: Used so Far: 4(2)+4(6) = 32: Remaining: 0: Last updated: August 27, 2005
Lewis structure of PO43 Science, Chemistry, Elements, Atoms
You can easily draw the Lewis dot structure of a phosphate ion with us using the simple guidelines given below. Steps for drawing the Lewis dot structure of [PO4]3- 1. Count the total valence electrons in [PO4]3- The Lewis dot structure of a molecule is referred to as a simplified representation of all the valence electrons present in it.
Po4 3 Lewis Structure slidesharetrick
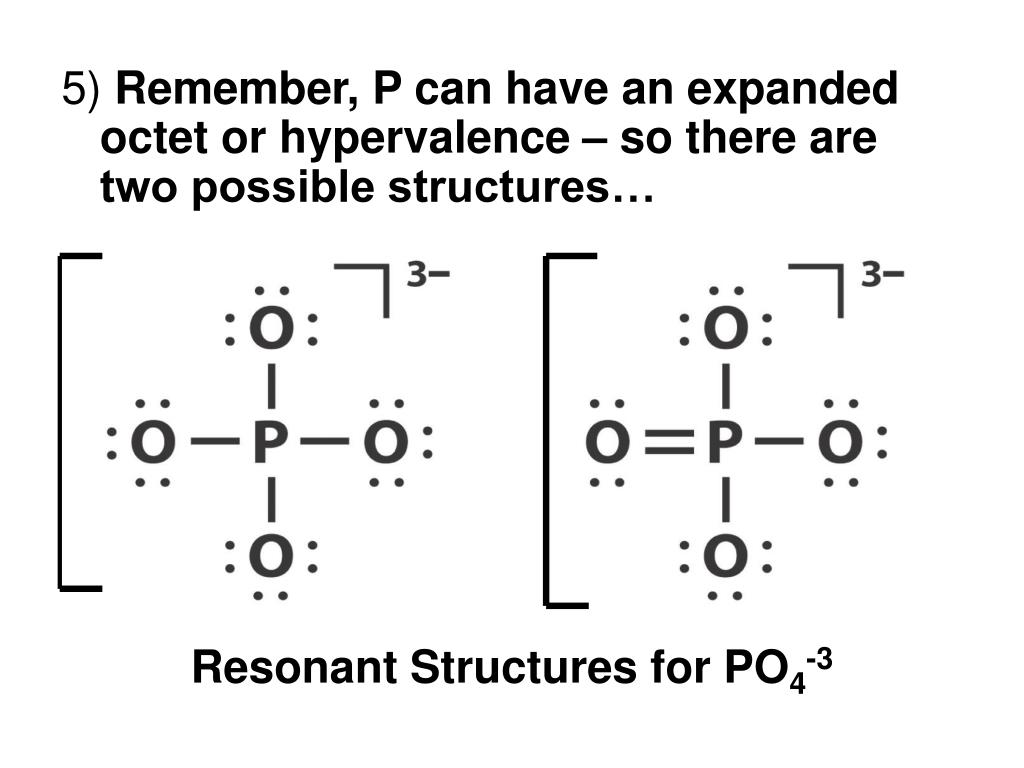
We start with a valid Lewis structure and then follow these general rules..more.more There are three resonance structures PO43- (Phosphate ion). We start with a valid Lewis structure.

PO4 3 Lewis Structure The Phosphate Ion YouTube
How to Draw Lewis Structure of PO4 3- I Easy & Quick Science Genius 26 subscribers Subscribe 0 2 views 9 months ago This video will explain how to draw a skeletal structure of phosphate ion..
Lewis dot structure for PO4 3 Phosphate ion YouTube
Let's do the Lewis structure for PO4 3-. Phosphorus has 5 valence electrons. Oxygen has 6, we've got 4 Oxygens. This negative 3 up here means we have three additional electrons. Five plus 24 plus 3 gives you 32. So those are our valence electrons. Put Phosphorus at the center and the Oxygens around it, all 4 of them.
Lewis structure of PO4 3 (Phosphate ion) YouTube
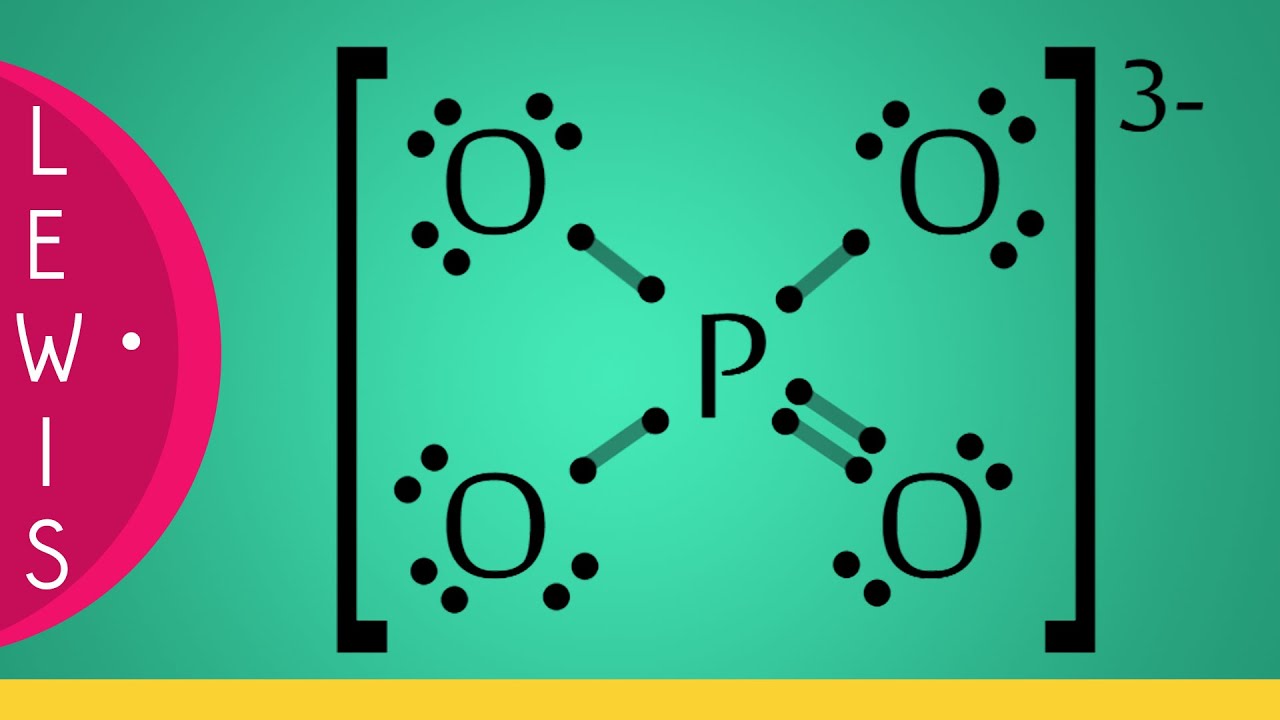
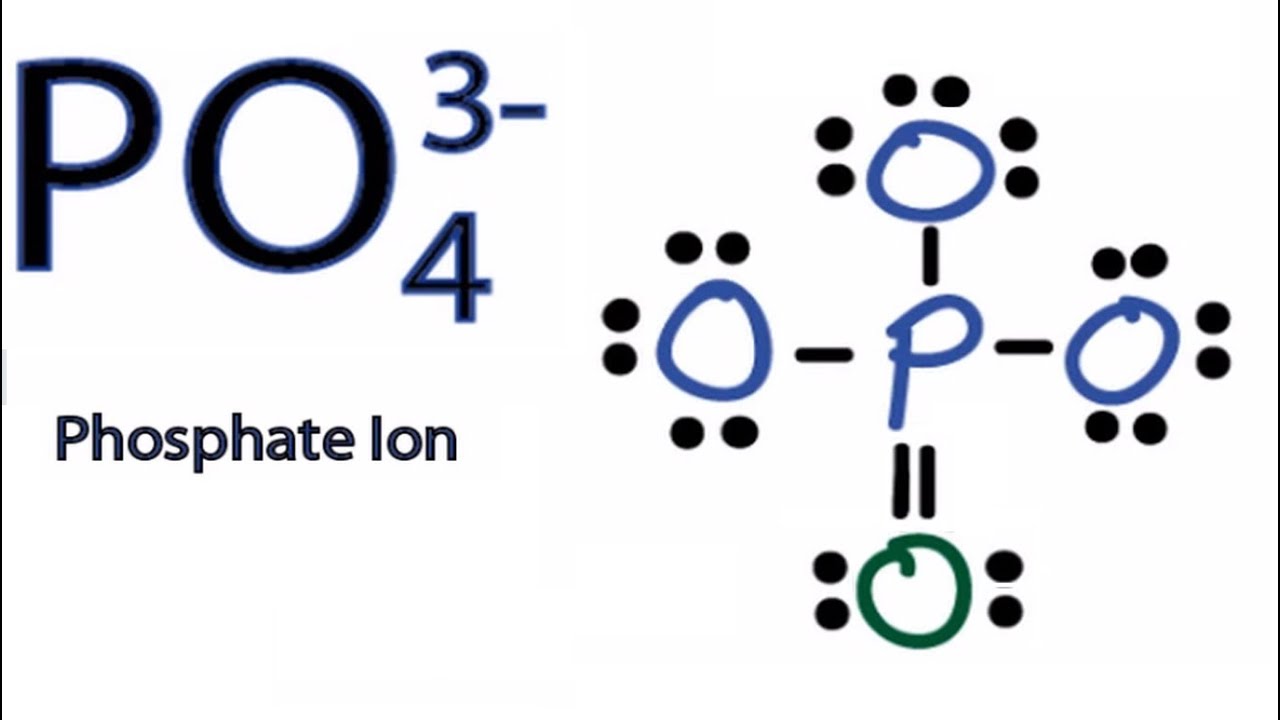
Lewis structure of PO43- ion (phosphate ion) contains one double bond and three single bonds between the Phosphorus (P) atom and Oxygen (O) atoms. The Phosphorus atom (P) is at the center and it is surrounded by 4 Oxygen atoms (O). Let's draw and understand this lewis dot structure step by step.
Strutture di Lewis • Ione Fosfato [PO4]3 YouTube
Share 1.6K views 1 year ago Lewis Structure PO4 3- is a chemical formula for Floroform. It consists of one sulphur atom and four oxygen atoms.
PPT Molecular Bonds Part II PowerPoint Presentation, free download
PO4 3- (phosphate ion) lewis structure has a Phosphorus atom (P) at the center which is surrounded by four Oxygen atoms (O). There is 1 double bond and 3 single bonds between the Phosphorus atom (P) and each Oxygen atom (O). There are 2 lone pairs on double bonded Oxygen atom (O) and 3 lone pairs on single bonded Oxygen atom (O).

Easy steps to draw LEWIS structure of PO4 3 (Phosphate ion) YouTube
1. Count valence electrons for PO4 3- 2. Put least electronegative atom (P) in centre. 3. Put one electron pair in each bond 4. Fill outer atoms with electrons first. 5. Move electrons so all.

Resonance Structures for PO4 3 (Phosphate ion) YouTube
Well, actually, you can draw the Lewis diagram with single bonds, and all the oxygens have 3 lone pairs. PO4^3- has 5+4x6+3 = 32 e-. The only reason that you might want to include a double bond is because of overly strict adherence to formal charges. By making one of the bonds a double bond, you reduce the formal charge on P from +2 to +1 and.
PO4 3 Lewis Structure How to Draw the Lewis Structure for PO43 YouTube
The Lewis Dot Structure for PO 4 3-: The phosphate anion (PO 4 3-) represents the fully ionized form of phosphoric acid (H 3 PO 4 ). This anion plays significant biochemical roles in living organisms. The Lewis structure can be drawn based on several general rules, and shows how valance electrons are used in the bonding between atoms.

Draw a single Lewis structure for the phosphate ion (PO4^3−), inwhich
A step-by-step explanation of how to draw the PO43- Lewis Dot Structure (Phosphate ion). For the PO4 3- structure use the periodic table to find the total number of valence electrons.

PO4 estructura de lewis urgenteeee Brainly.lat
In the Lewis structure of PO43-, P forms single bonds with 3 oxygen atoms and forms a double bond with one oxygen atom. These oxygen atoms carry a charge of -1. Let us now look that steps required for drawing a Lewis structure:- 1. Counting the total number of valence electrons of the molecule. 2. Locating the central atom of the molecule. 3.