/PeriodicTableCharge-WBG-56a12db23df78cf772682c37.png)
Periodic Table With Common Ionic Charges
acetate PERIODIC TABLE OF IONS arsenate arsenite benzoate borate bromate carbonate chlorate chlorite chromate cyanate cyanide dichromate CH3COO- AsO4 3- AsO3 3- C6H5COO - BO3 3- BrO3 - CO3 2- ClO3 - ClO2 - CrO4 2- CNO- CN- Cr2O7 2- oxalate perchlorate periodate permanganate peroxide phosphate pyrophosphate sulfate.

Naming Simple Ionic Compounds Pathways to Chemistry
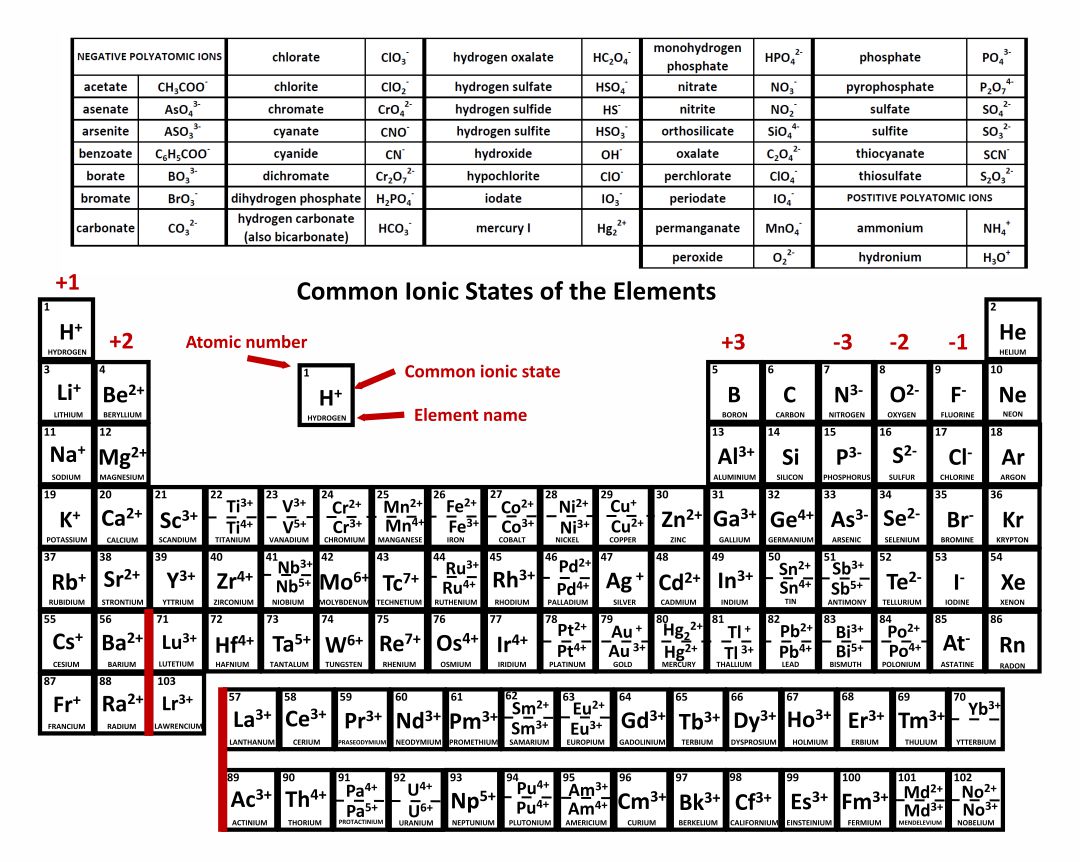
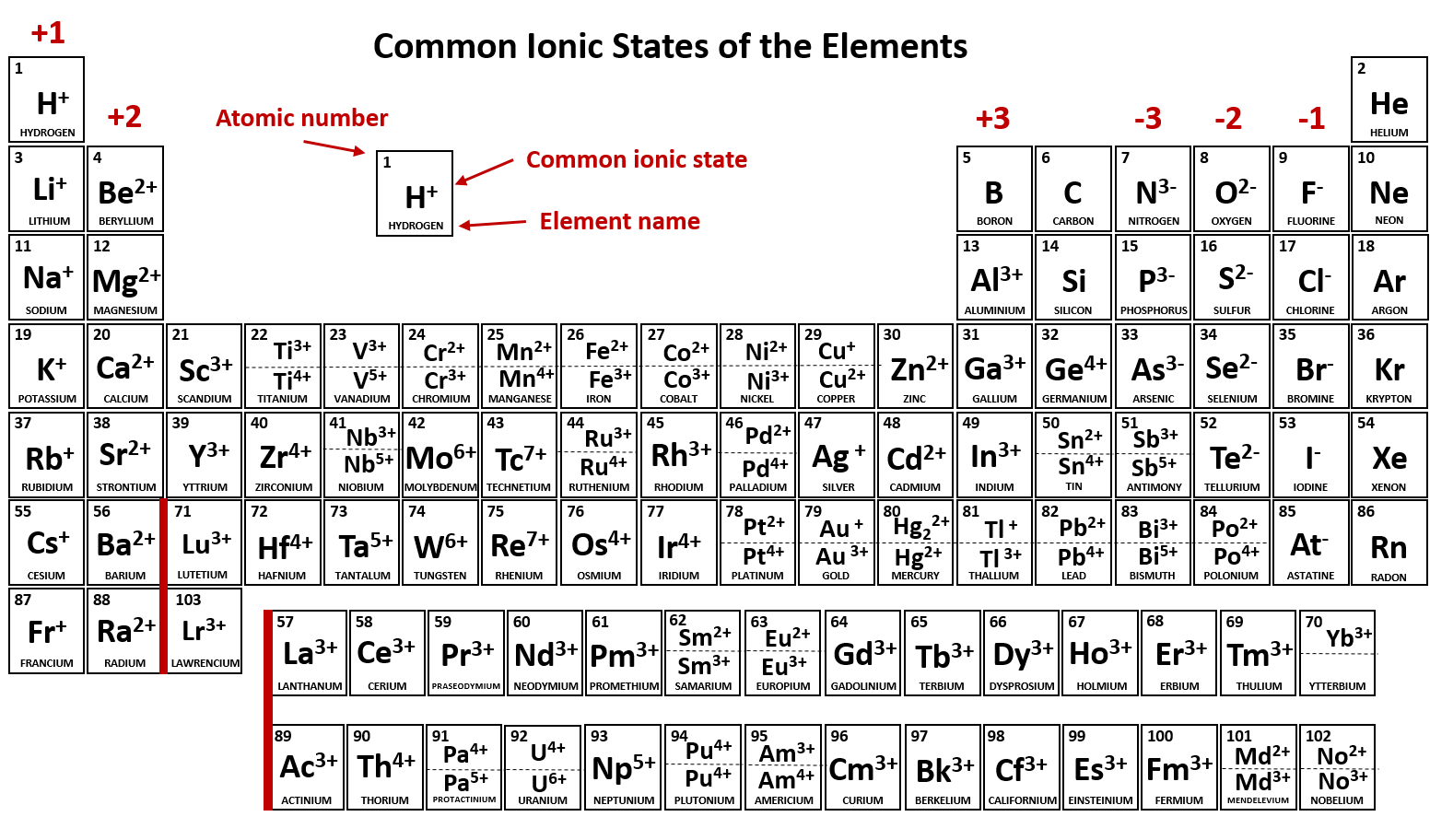
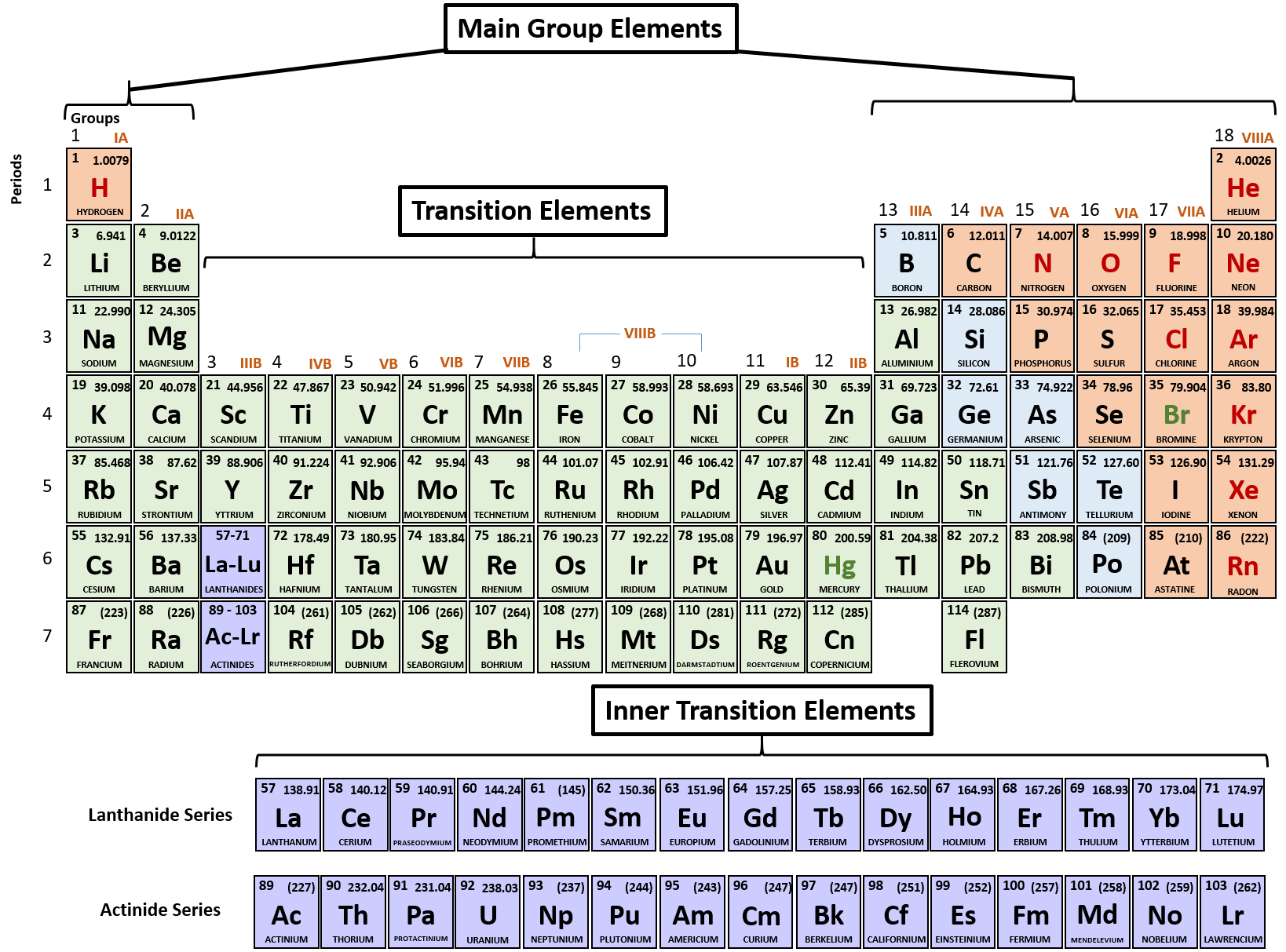
As you can see from the partial table shown above the Groups of the periodic table each form a unique charge of ion. The Natural formation of ions is: Group 1 elements form +1 ions.. This is why it is so important for you to know the locations of the metals and non-metals in the periodic table. Polyatomic Ions.

Common Ion Charges Periodic Table Periodic Table Timeline
Note the usefulness of the periodic table in predicting likely ion formation and charge (Figure \(\PageIndex{2}\)). Moving from the far left to the right on the periodic table, main-group elements tend to form cations with a charge equal to the group number. That is, group 1 elements form 1+ ions; group 2 elements form 2+ ions, and so on.
10 Best Printable Periodic Table Of Ions PDF for Free at Printablee
acetate PERIODIC TABLE OF IONS arsenate arsenite benzoate borate bromate carbonate chlorate chloride chlorite chromate cyanate cyanide dichromate CH3COO- AsO4 3- AsO3 3- C6H5COO - BO3 3- BrO3 - CO 3 2- ClO3 - Cl- ClO2 - CrO4 2- CNO- CN- Cr2O7 2- oxalate perchlorate periodate permanganate peroxide phosphate.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
In this activity, students will use the online periodic table, ptable.com, to investigate a number of chemistry concepts. Students will use this online resource to explore information about the elements, including historical data, physical properties, periodic trends and more.
4.08 Ions Chemistry LibreTexts
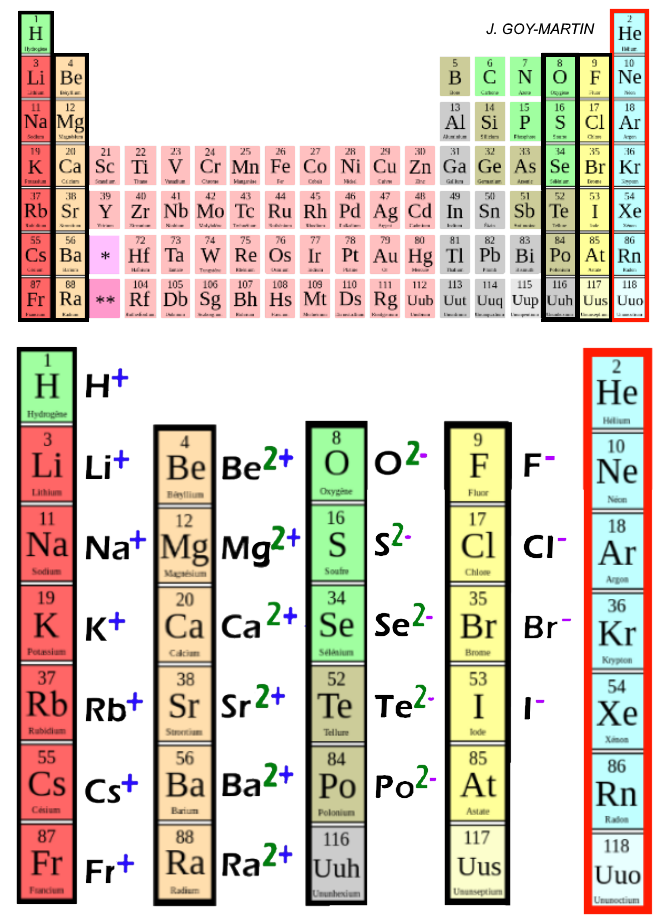
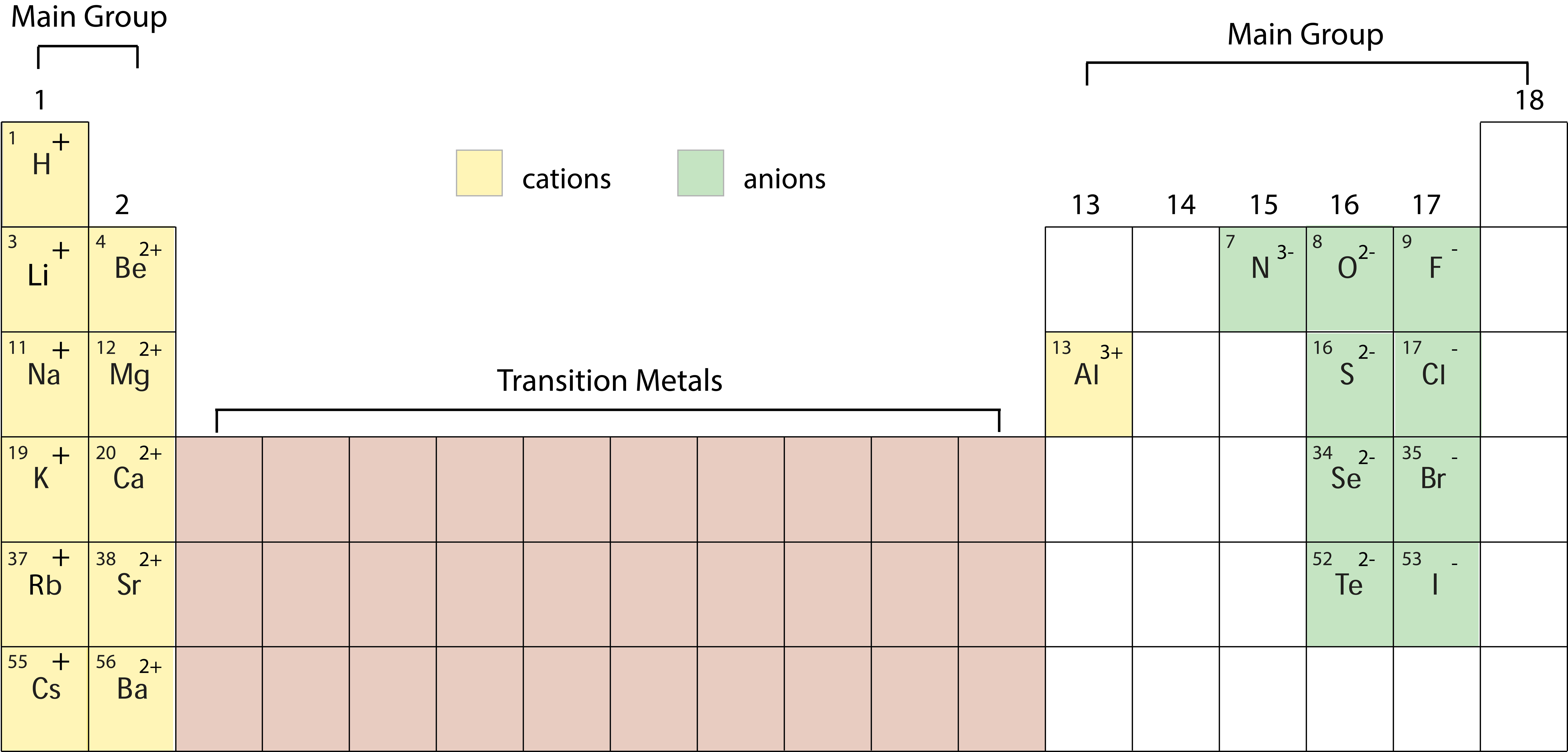
Cations are positively charged ions formed when neutral atoms lose electrons; anions are negatively charged ions formed when neutral atoms gain electrons. It is possible to predict the charges of common monatomic ions by looking at the group numbers on the periodic table.
10 Best Printable Periodic Table Of Ions PDF for Free at Printablee
They usually react with ions of opposite charge to form neutral compounds. For example, positive sodium ions and negative chloride ions react to form the neutral compound sodium chloride, commonly known as table salt. This occurs because oppositely charged ions attract each other. Ions with the same charge, on the other hand, repel each other.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Main group elements (the elements in groups 1, 2, and 13-18 of the periodic table) typically form ions of only one charge. The diagram below shows the common charge of ions in different groups. A periodic table showing the common charges of ions formed by elements in different groups. Group 1, the first column, has a column charge of 1+. Group.

Ions
Note the usefulness of the periodic table in predicting likely ion formation and charge (Figure \(\PageIndex{2}\)). Moving from the far left to the right on the periodic table, main-group elements tend to form cations with a charge equal to the group number. That is, group 1 elements form 1+ ions; group 2 elements form 2+ ions, and so on.
CH103 CHAPTER 4 Ions and Ionic Compounds Chemistry
Table of Ions/Charges of Common Elements. Below given is a chart of the most common charges for chemical element atoms. It can be used to predict whether or not an atom can bond with another atom.. Which group in the periodic table cannot form ions? Since the Group 8A elements have a full octet of eight valence electrons in their highest.

Ionic Compounds Periodic Table
Periodic Chart of Ions IA VIIIA 1 H+ hydrogen IIA IIIA IVA VA VIA VIIA 2 He helium 3 Li+ lithium 4 Be2+ Beryllium 5 B boron 6 C carbon 7 N3-nitride 8 O2-oxide 9 F-fluoride 10 Ne neon 11 Na+ sodium 12. Table of Polyatomic Ions acetate CH 3COO-dichromate Cr 2O 7 2-dihydrogen phosphate H 2PO 4-ammonium NH 4 + cyanide CN-silicate SiO 3 2.
PreChemistry
Interactive periodic table showing names, electrons, and oxidation states. Visualize trends, 3D orbitals, isotopes, and mix compounds. Fully descriptive writeups.

Ion Table Periodic Table And Ionic Charges I As Periodic Table With
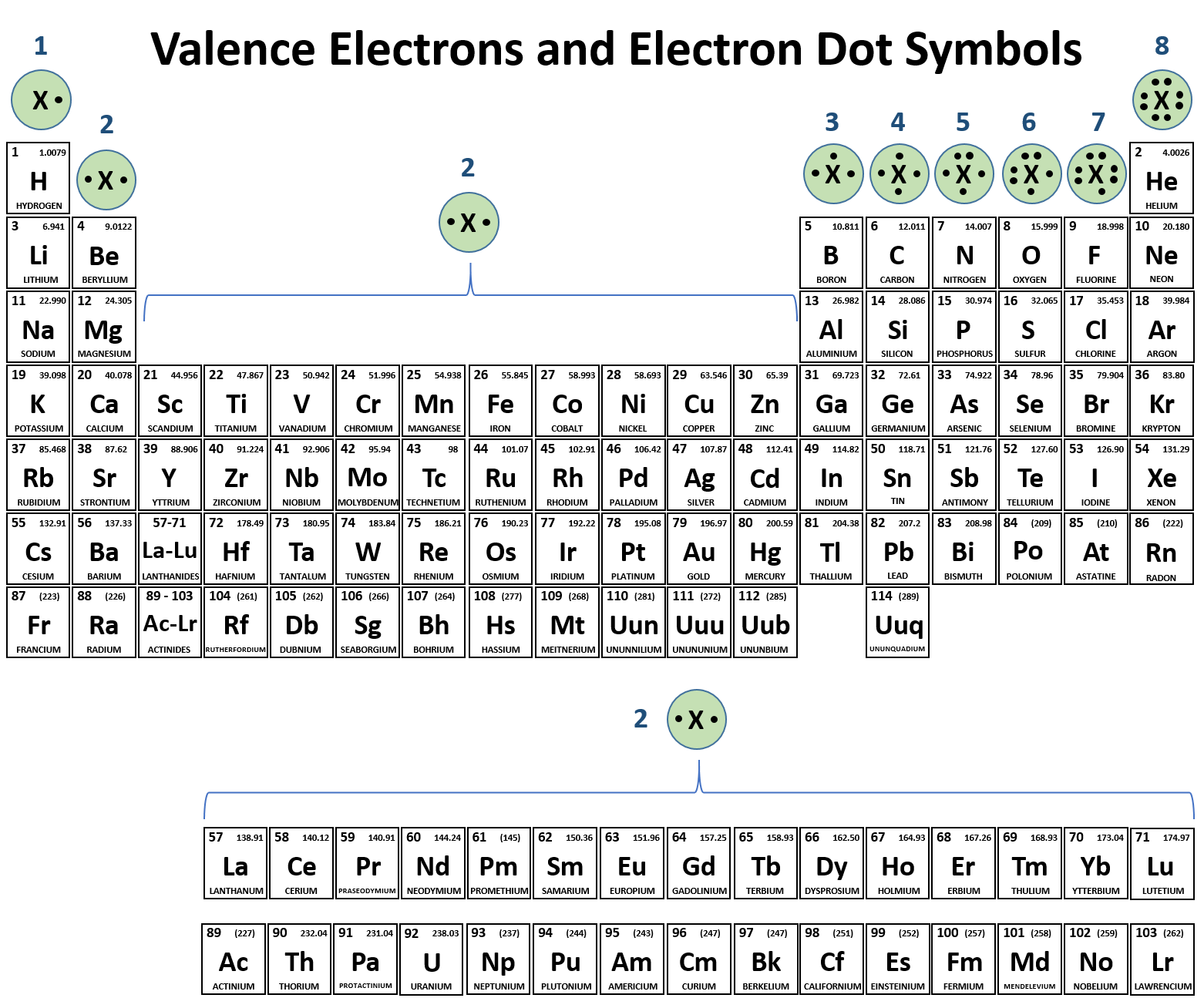
We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table.

Periodic Table Of Ions Printable Printable Word Searches
Note the usefulness of the periodic table in predicting likely ion formation and charge (Figure 2.29). Moving from the far left to the right on the periodic table, main-group elements tend to form cations with a charge equal to the group number. That is, group 1 elements form 1+ ions; group 2 elements form 2+ ions, and so on.

periodic table of ions printable Periodic Chart of Ions PDF
For example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. On the other side of the periodic table, the next-to-last column, the halogens, form ions having a 1− charge.

Chem Ions Scientific Tutor
Interactive periodic table with up-to-date element property data collected from authoritative sources. Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game!