
Pharmacovigilance and ICH guidlines
ICH Guidelines. All Guidelines; Quality Guidelines; Safety Guidelines; Efficacy Guidelines; Multidisciplinary Guidelines; Index of Guidelines; ICH Standards. MedDRA; CTD; Electronic Standards (ESTRI) Other Work Products. Reflection Papers & Discussion Groups; Consideration Documents; CIOMS Glossary of ICH Terms & Definitions; Meetings .

11. ROLE AND RESPONSIBILITIES OF CLINICAL TRIAL PERSONNEL AS PER ICH GCP PHARMD GURU
This table lists ICH guidelines that have recently been finalised at ICH and are pending implementation or have either been implemented by Health Canada in the last 12 months.. Pharmacovigilance Planning. 2009/02/16. 09-103644-626.. Good Clinical Practice. 2019/04/03. 19-105427-311. E7: Guideline - Studies in Support of Special.

ICH pharmacovigilance planning, an efficacy guideline
The ICH Harmonised Guideline was finalised under Step 4 in October 1994. This document gives standard definitions and terminology for key aspects of clinical safety reporting. It also gives guidance on mechanisms for handling expedited (rapid) reporting of adverse drug reactions in the investigational phase of drug development. Date of Step 4:

ICH Guidelines for Pharmacovigilance
Safety Guidelines ICH has produced a comprehensive set of safety guidelines to uncover potential risks like carcinogenicity, genotoxicity and reprotoxicity. A recent breakthrough has been a non-clinical testing strategy. ( more) Efficacy Guidelines

ICH pharmacovigilance planning, an efficacy guideline
You can explore in the below table the index of all ICH Guidelines, finalised or under development, on the topics of Quality, Safety, Efficacy and Multidisciplinary. Please select first the relevant topic. You can then search by ICH Step status, date, and/or by keyword.

Good Clinical Practices Guideline ICHGCP Principles of GCP Hindi Pharmacovigilance
Guideline on good pharmacovigilance practices (GVP) - Module VI EMA/542040/2014 (superseded version) Page 2/90 . VI.C.2.2.3.. in the ICH-E2A and ICH-E2D guidelines. 1. should also be adhered to ; they are included as well in this chapter. VI.A.2.1. Adverse reaction .

ICH Guidelines for Pharmacovigilance
1.1 Objective. This guideline is intended to aid in planning pharmacovigilance activities, especially in preparation for the early postmarketing period of a new drug (in this guideline, the term "drug" denotes chemical entities, biotechnology-derived products, and vaccines).

ICH Guidelines for Pharmacovigilance
Efficacy Guidelines The work carried out by ICH under the Efficacy heading is concerned with the design, conduct, safety and reporting of clinical trials. It also covers novel types of medicines derived from biotechnological processes and the use of pharmacogenetics/genomics techniques to produce better targeted medicines.
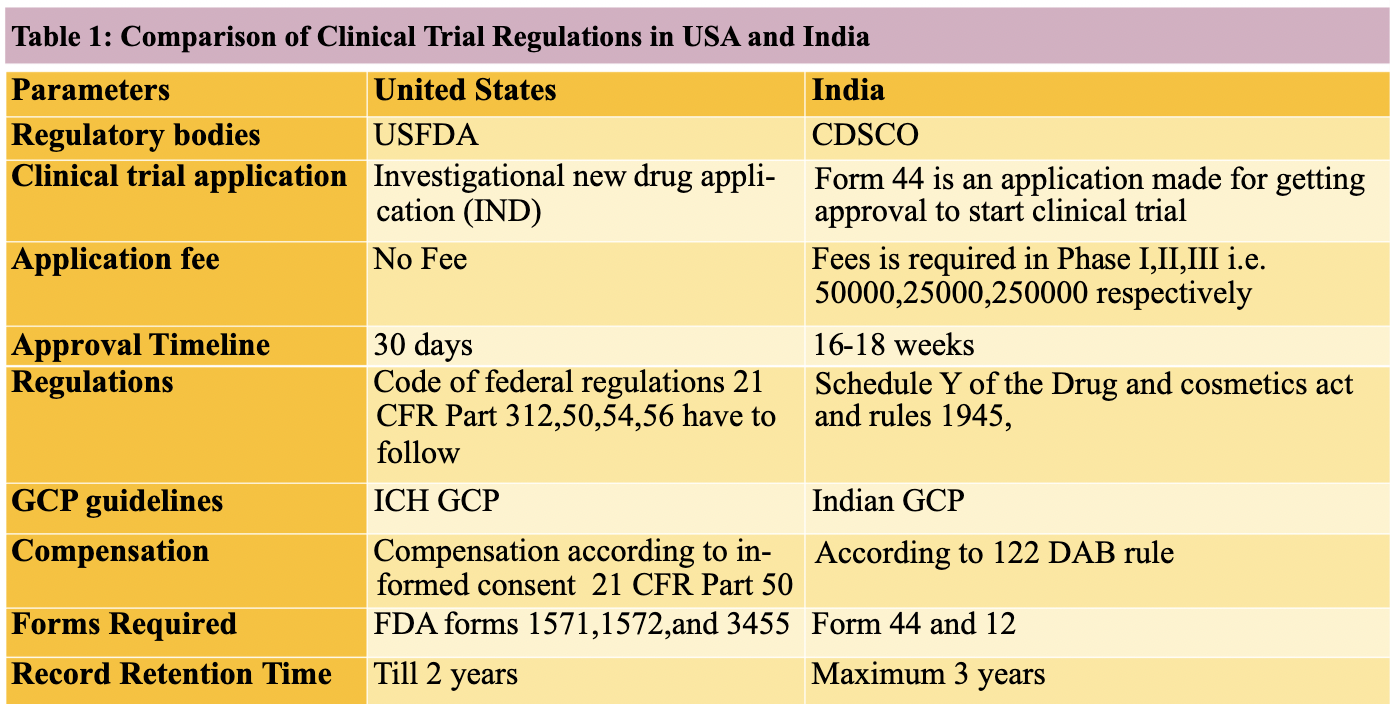
Difference between ICH GCP and schedule Y CCRPS
1.9 Deviations from procedures relating to pharmacovigilance activities should be documented. 1.10 When part or all pharmacovigilance activities are performed by a third party, MAH and importers should review procedures to ensure that procedures are adequate and compliant with applicable requirements stated in the Food and Drug Regulations.
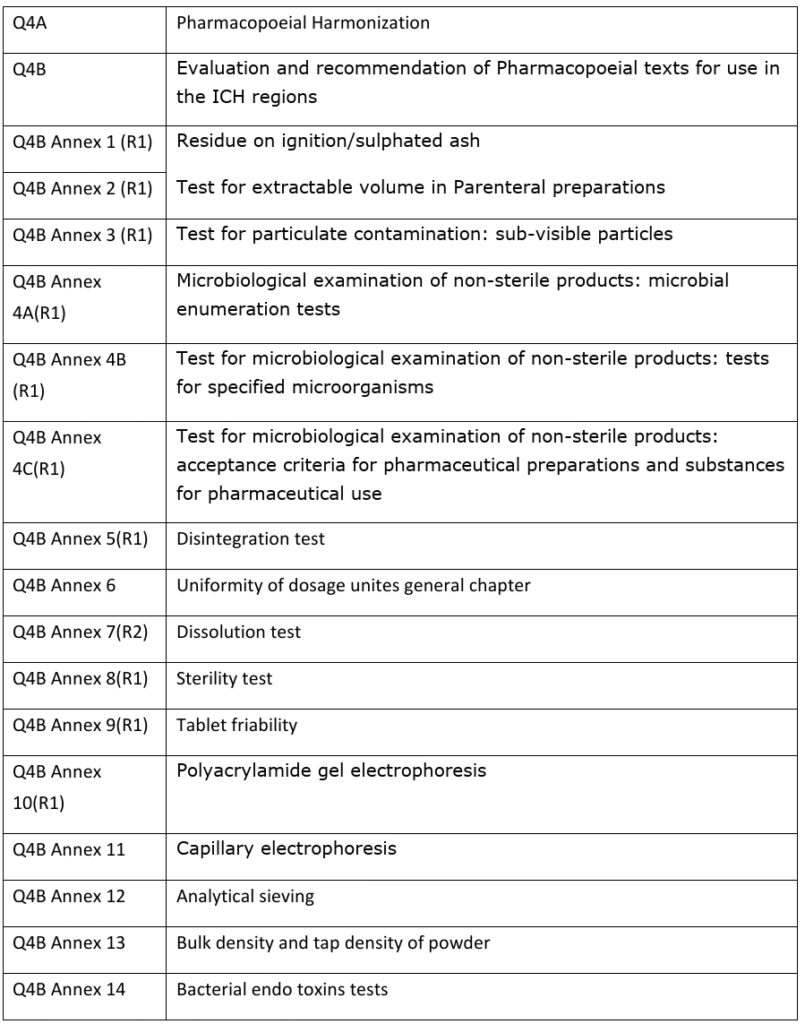
ICH Guidelines in Pharmaceutical (updated) » Pharmaguddu
- New guidance on the electronic submission modalities of ICSRs under the new ICH-E2B(R3) format; - New guidance on the management of individual reports of off-label use, based on the Reflection Paper on Collecting and Reporting Information on Off-label Use in Pharmacovigilance (EMA/293194/2016), published for public consultation in 2016;

ICH GCP
Good pharmacovigilance practices (GVP) are a set of measures drawn up to facilitate the performance of pharmacovigilance in the European Union (EU). GVP apply to marketing-authorisation holders, the European Medicines Agency (EMA) and medicines regulatory authorities in EU Member States.

ICH pharmacovigilance planning, an efficacy guideline
ICH E10 Choice of control group in clinical trials. ICH E11 (R1) step 5 guideline on clinical investigation of medicinal products in the pediatric population - Scientific guideline. ICH guideline E11A on pediatric extrapolation - Step 2b. ICH guideline E17 on general principles for planning and design of multi-regional clinical trials.

Role of ICH GCP in Clinical Trials JLI Blog
08 - 11 February 2024 AMIA (American Medical Informatics Association) United States The SCRS Oncology Site Solutions Summit 10 - 11 April 2024 The Society for Clinical Research Sites (SCRS) United States PCC 2024 — Pharmaceutical Compliance Congress 16 - 18 April 2024 Informa United States SCDM 2024 EMEA Conference 17 - 19 April 2024

ICH Guidelines for Pharmacovigilance
In addition, these Regulations integrate the principles of Good Clinical Practices (GCP) as described by the International Conference on Harmonization (ICH). The inspection of clinical trials will be initiated in close collaboration with the Therapeutic Products Directorate (TPD) and the Biologic and Radiopharmaceutical Drugs Directorate (BRDD).

The Importance of ICH GCP CCRPS
This document provides guidance on planning pharmacovigilance activities, especially in preparation for the early postmarketing period of a new medicinal product. It applies to chemical entities, biotechnology -derived products and vaccines.

CCRB ICH GCP Knowledge Test
This International Conference on Harmonization (ICH) guidance addresses the choice of control group in clinical trials, discussing five principal types of controls, two important purposes of.