
Draw the resonance structure of 1) CO3 2 2) Benzene 3) CO2 4) O3 Pls Answer It ASAP . I need
I quickly take you through how to draw the Lewis Structure of CO3 2- (Carbonate Ion). I also go over the resonance, hybridization, shape and bond angle.
PPT Lecture 11 VSEPR Theory, Molecular Shape PowerPoint Presentation ID6303965
1.8K 161K views 3 years ago This chemistry video tutorial explains how to draw the lewis structure of CO3 2- also known as the carbonate ion. This video discusses the resonance structure of.
CO23. Solutions to Selected Problems, CO19 Chemistry LibreTexts
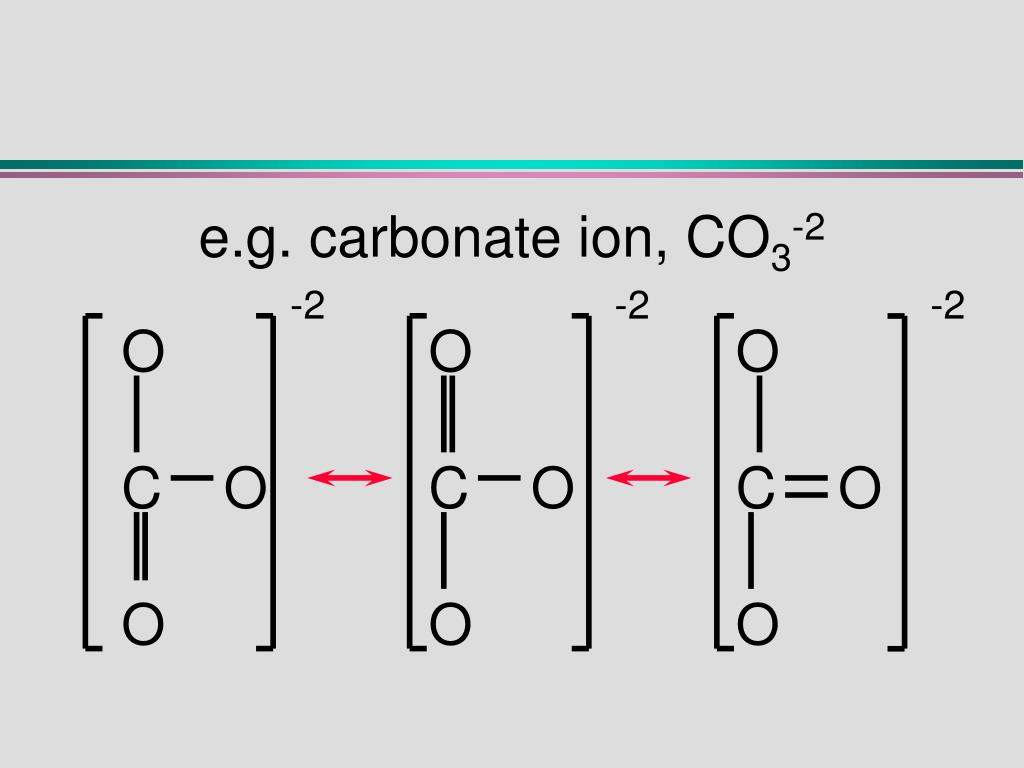
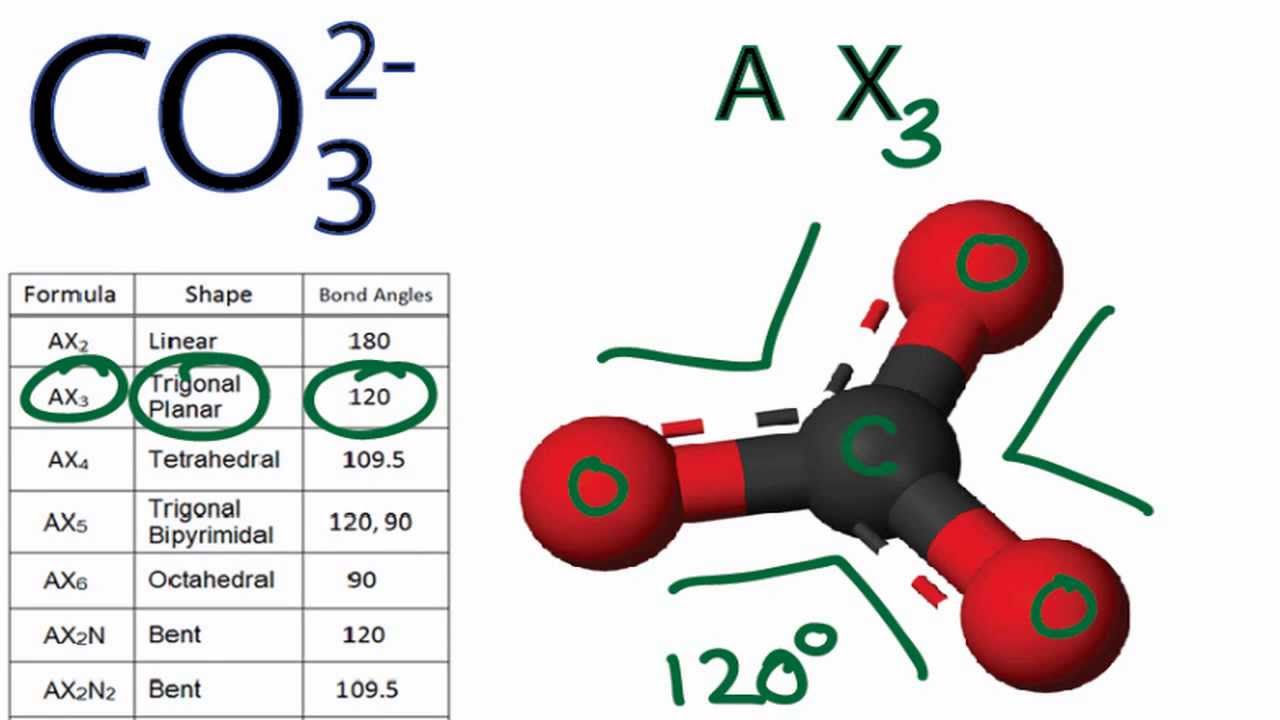
Carbonate, \(\ce{CO3^2-}\), is a common polyatomic ion found in various materials from eggshells to antacids. What are the electron-pair geometry and molecular structure of this polyatomic ion? Answer. The electron-pair geometry is trigonal planar and the molecular structure is trigonal planar. Due to resonance, all three C-O bonds are identical.

Co3 2 Molecular Geometry
Figure 5.2.9 5.2. 9: (a) H2O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry. (b) Two of the electron regions are lone pairs, so the molecular structure is bent. Exercise 5.2.3 5.2. 3. The hydronium ion, H 3 O +, forms when acids are dissolved in water.
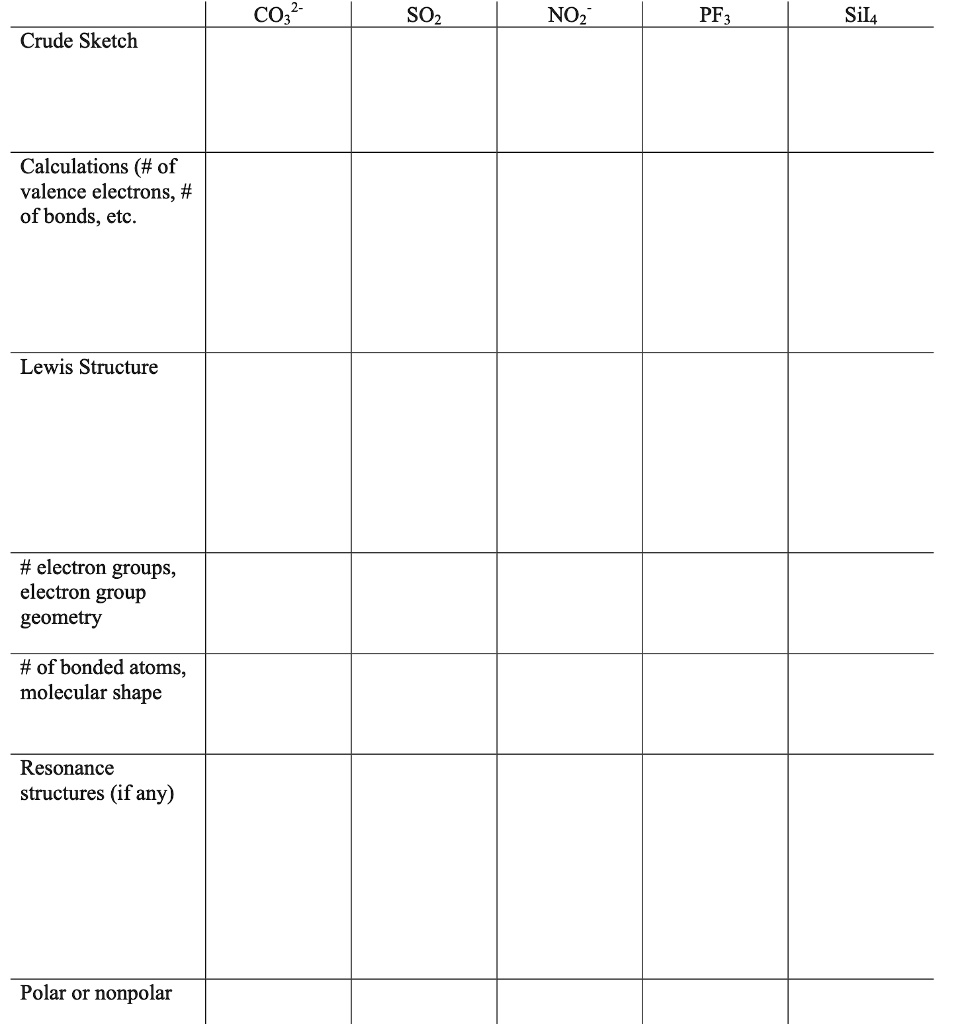
SOLVED CO3 SOz NOz PFz Sil4 Crude Sketch Calculations ( of valence electrons, of bonds, etc
329 Share 110K views 10 years ago A quick explanation of the molecular geometry of CO3 2- including a description of the CO3 2- bond angles..more.more A quick explanation of the.

draw the resonance structure of the following substance. dickvandykemarypoppinsbanker
10.3: VSEPR Geometry. To use the VSEPR model to predict molecular geometries. To predict whether a molecule has a dipole moment. The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons.

What is the molecular and electron geometry of \ce{CO3^{2} Quizlet
Lewis structure of CO3 2- contains one double bond and two single bonds between the Carbon (C) atom and Oxygen (O) atom. The Carbon atom (C) is at the center and it is surrounded by 3 Oxygen atoms (O). Both the single bonded Oxygen atoms (O) have -1 formal charge. Let's draw and understand this lewis dot structure step by step.

CO32 Lewis Structure How to Draw the Lewis Structure for CO3 2 (Carbonate Ion) YouTube
Step - 8 Last is to determine shape, hybridization and bond angle of CO32- lewis structure. CO32- lewis structure.. Carbonate (CO32-) ions have 2- negative formal charge and also it has quite sufficient lone electron pairs present on three O atoms out if which two O atoms have -1 negative charge. Thus it can easily gain or accepts H+ ions.

[Download 35+] Possible Resonance Structures For Co32
The Lewis structure of H 2 O indicates that there are four regions of high electron density around the oxygen atom: two lone pairs and two chemical bonds: Figure 4.3.9 4.3. 9. Thus, the electron-pair geometry is tetrahedral and the molecular structure is bent with an angle slightly less than 109.5°.
Co Formulacion SEO POSITIVO
Hello Guys!CO32- ion comprises one Carbon atom and three Oxygen atoms along with two additional electrons. In this video, we find out the molecular geometry.

How To Draw Resonance Structures Foreversalary
3812-32-6 Carbonate ions Karbonat View More. Molecular Weight 60.009 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Parent Compound CID 767 (Carbonic Acid) Dates Create: 2005-08-08 Modify: 2024-01-06 Description Carbonate is a carbon oxoanion. It is a conjugate base of a hydrogencarbonate. ChEBI

SOLVED Draw the Lewis structure of CO. What is the electron geometry, molecular shape, and
A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure (Carbonate ion). For the CO3 2- structure use the periodic table to find the total number of valence electrons.

Lewis dot structure CO3 2 How to draw Lewis structures for ions Carbonate Ion Chemical
CO32- Geometry and Hybridization - Chemistry Steps Examples Geometry CO32- Geometry and Hybridization There are 4 + 3×6 + 2 = 24 electrons. The carbon goes in the middle, and the oxygens take 6 electrons each as three lone pairs: The carbon lacks an octet, so we use a lone pair from one oxygen to make a double with it.

Lewis dot structure for NF3.and CO3^2_ Brainly.in
Lewis structure of carbonate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of CO 32-. After finishing the lewis structure of CO 32-, there should be a -2 charge and it should be stabile structure. You will learn about these facts in this tutorial. Carbonate ion | CO 32-

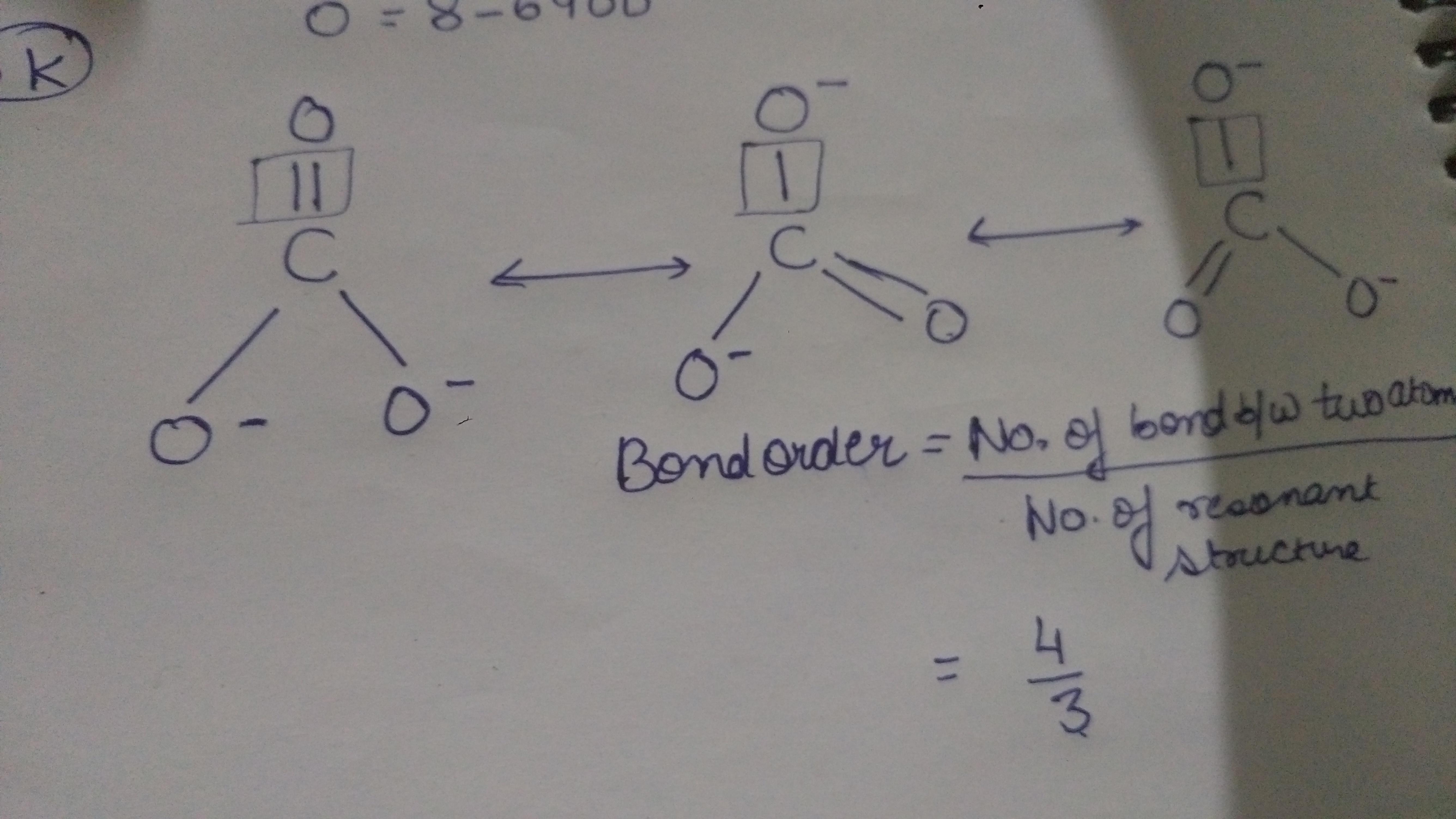
How to calculate bond order of co3^ 2? Brainly.in
CO3 2- Lewis Structure Step-by-Step Guide 1. Determine the total number of valence electrons In the carbonate ion (CO3^2-), carbon (C) contributes 4 valence electrons, while each oxygen (O) atom contributes 6 valence electrons. Since there are three oxygen atoms, the total number of valence electrons is:
¿Cuál es la estructura de Lewis de Co3 2?
1. Count the total valence electrons in [CO3]2- The Lewis dot structure of a molecule is referred to as a simplified representation of all the valence electrons present in it. Therefore, the very first step while drawing the Lewis structure of [CO 3] 2- is to count the total valence electrons present in the concerned elemental atoms.