PPT Physical Science PowerPoint Presentation ID2132853
12 Key Differences (Compound vs Mixture) Examples of Compound Water Examples of Mixture Crude Oil References and Sources Compounds are those substances that are composed of many identical molecules and consist of two or more atoms from more than one element linked together by chemical bonds.

Compound and mixture; Examples and differences To The Geeks
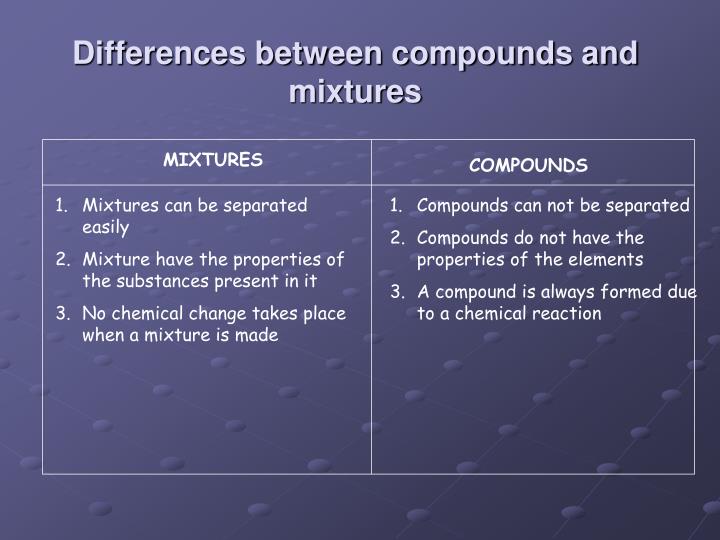
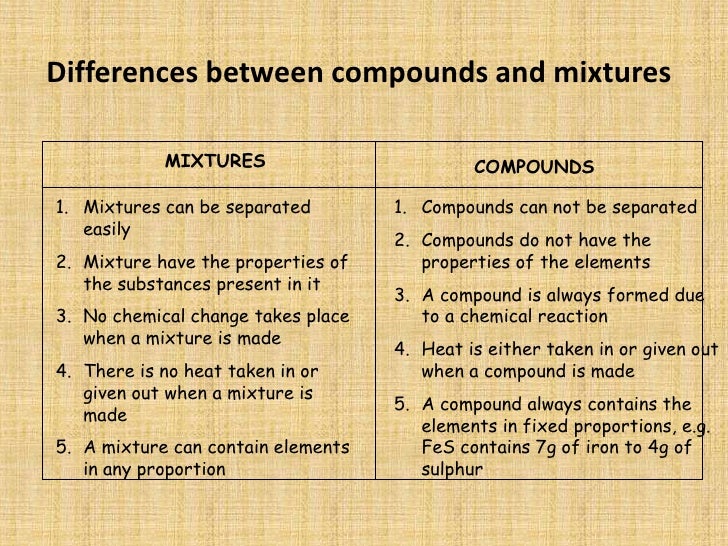
An impure substance made from different elements or compounds mixed together that are not chemically joined. Mixtures can usually be separated by physical techniques such as filtering and.

Difference between Mixture and Compound in Chemistry YouTube
Answer1: One difference between compound and mixture is that the compound has a definite unchangeable melting or boiling point while the mixture is devoid of it. Question 2: Give some examples of compound and mixture? Answer 2: Some examples of compounds are salt, baking soda, and methane.

Difference between compound and mixture Compound vs Mixture YouTube
The important difference between Mixtures and Compounds After highlighting the differences between mixtures and compounds, we will be giving a brief description of mixtures and compounds. Mixture When we mix two or more substances together, whose ratio is not fixed such that no chemical reaction takes place, the substance formed is a mixture.
Difference Between Mixture And Compound cloudshareinfo
A compound consists of different kind of atoms which are chemically bonded. A mixture is made up of two or more different kinds of substances (atoms, molecules or compounds) physically intermingled. The main difference between Compound and Mixture is that compounds are chemically bonded whereas mixtures are not. This article explains, 1.

Difference Between Compound and Mixture with Definition
A substance formed by physically mixing two or more different materials in a random proportion without any molecular bonding is referred to as a mixture. Properties of Mixtures The components of mixtures have no chemical bonding between their respective molecules.

what are the difference between mixture and compound Brainly.in
Elements And Compounds 45,756 The differences between mixtures and compounds are tabulated below. Difference Between Compound and Mixture These were the main mixtures and compounds differences that are crucial for not only examinations but also for competitive exams.

15 Must Read Difference Between Compound and Mixture Core Differences
Comparison Chart Definition of Compound Compound means a substance formed as a blend of various elements chemically in a certain proportion, by weight. It is entirely new substance, which possesses properties different from that of its constituent substances. For example - Water, salt, carbon dioxide, sodium chloride, etc.
The Difference Between Alloys and Composites (and Compounds
Let's take a closer look at each of these concepts! A mixture is a physical combination of two or more substances, while a compound is a substance made up of atoms of one element that are chemically bonded to atoms of another element. Explain it to a child

images of elements compounds and mixtures differences Brainly.in
The key difference between compounds and mixtures is that a compound is made up of molecules, each of which is composed of two or more different types of chemically bound atoms. While the mixture is a combination of two or more elements or compounds that are combined physically rather than chemically.

Difference Between Compound and Mixture Compare the Difference
What's the difference between Compound and Mixture? Compounds are pure substances. They are made from the same types of molecules. Each molecule of a compound is made from two or more different kinds of atoms that are chemically bonded. Mixtures are made of two or more substances — elements or compounds — t.

1.8 Elements, compounds, mixtures Chemistry
A mixture refers to a physical combination of two or more substances, where no reaction occurs. Because only a physical change occurred, a mixture can separate back into its original components. For example, saltwater is a mixture. When you boil it, the water evaporates while the salt stays behind.

difference between compound and mixture Brainly.in
The main difference between a compound and a mixture is the way the constituent substances are combined. In a compound, the constituent elements are chemically bonded together in a fixed ratio, forming a new substance with distinct properties. In a mixture, the constituent substances are simply physically mixed together, but they retain their.

Chemistry 9th Chapter 1 Difference between Compound and Mixture
1. Matter is made up of atoms that are indivisible and indestructible. 2. All atoms of an element are identical. 3. Atoms of different elements have different weights and different chemical properties. 4. Atoms of different elements combine in simple whole numbers to form compounds. 5. Atoms cannot be created or destroyed.

6 Differences between Compounds and Mixtures with examples
Particle diagrams - mixtures Test your knowledge Quiz Test questions Key points Everything in the known universe is made up of the elements found on the periodic table. There are over 100.

two different types of mixtures are shown in the table, one is labeled
Salt is a compound made up of two elements, sodium and chlorine. However, it can be found in nature as a mixture. For example, sea salt is a mixture of salt and other minerals found in seawater. While salt is a compound when it is pure, it can also be considered a mixture in certain contexts. 3.