
Is NF3 Polar or Nonpolar (Nitrogen Trifluoride) YouTube
In NF3 we do not have linear geometry. Due to the asymmetry resulting from the presence of a lone pair on the central nitrogen atom, we get polarity. NF3 is a polar molecule. You must also go through the detailed article written on the polarity of NF3. Note: NF3 is quite less polar than NH3 (since the net dipole moment is less).

BF3 and nf3 both molecules are covalent BF3 is nonpolar and nf3 is
NF3 is a covalent (polar covalent) compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, N is a nonmetal and F is also a nonmetal. So when they combine, it forms a covalent compound. Well, now you have got to know that NF3 is a covalent compound, but let me explain the in-depth reason why.

Bf3 and nf3 both molecules are covalent but bf3 is non polar and nf3 is
BF3 (Boron Trifluoride): This molecule is nonpolar. Boron and Fluorine have different electronegativities, but the shape of BF3 is trigonal planar, which allows for the dipoles to cancel out. Answer. 5. BrCl3 (Bromine Trichloride): This molecule is polar. Bromine and Chlorine have different electronegativities, creating a dipole moment.

Answered 5. How many of the following contain at… bartleby
Nitrogen trifluoride NF 3 is a polar molecule. It consists of polar N-F bonds due to the electronegativity difference of 0.94 units between nitrogen and a fluorine atom. Nitrogen trifluoride NF 3 has an asymmetrical trigonal pyramidal shape with a 101.9 ° bond angle due to a lone pair of electrons on the central N-atom in NF 3.

BF3 and NF3 both are covalent compounds but NF3 is polar whereas BF3 is
In a nonpolar covalent bond, the distribution of electrical charge is balanced between the two atoms. Figure 4.3.2 4.3. 2: A nonpolar covalent bond is one in which the distribution of electron density between the two atoms is equal. The two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density.

Is NF3 Polar or Nonpolar? Techiescientist
DO NOT FORGET TO SUBSCRIBE!LinkedIn: https://www.linkedin.com/in/kevan-j-e.Snapchat: https://www.snapchat.com/add/kravonoInstagram: https://www.instagram.c.

Is NF3 (Nitrogen trifluoride) Polar or NonPolar? YouTube
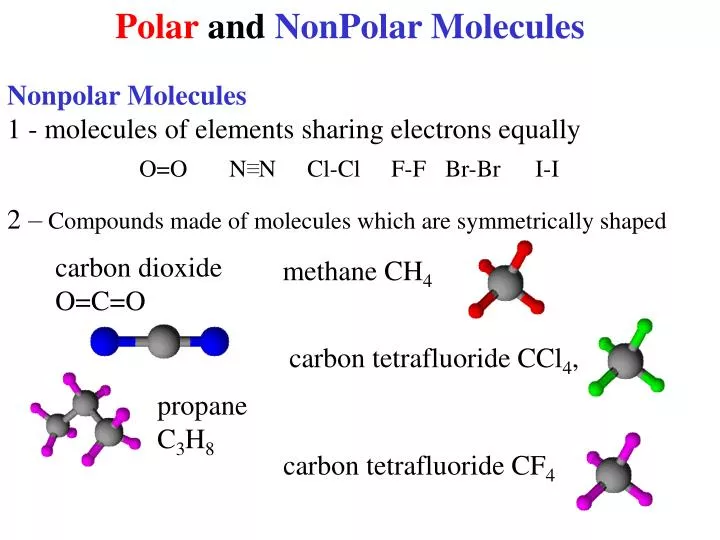
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms.
Ch4 Polar Or Nonpolar / What Is A Nonpolar Covalent Bond? Science
So, is NF3 Polar or Nonpolar? NF3 is polar in nature due to the presence of lone pair on nitrogen atom causing a distorted shape of NF3 molecule and the difference between the electronegativity of fluorine(3.98) and nitrogen(3.04) causes polarity in N-F bonds and result in a non zero dipole moment of the entire molecule.

Nitơ trifluoride NF3 khí đặc biệt cho Plasma
High reactivity: Nf3 is highly reactive and can easily react with various substances, including water, metals, and organic compounds. Non-polarity: Nf3 is a non-polar molecule due to the symmetry in the arrangement of its atoms. This characteristic affects its behavior in different chemical reactions.

Lectura Enlaces covalentes Biología I Association LEA
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.
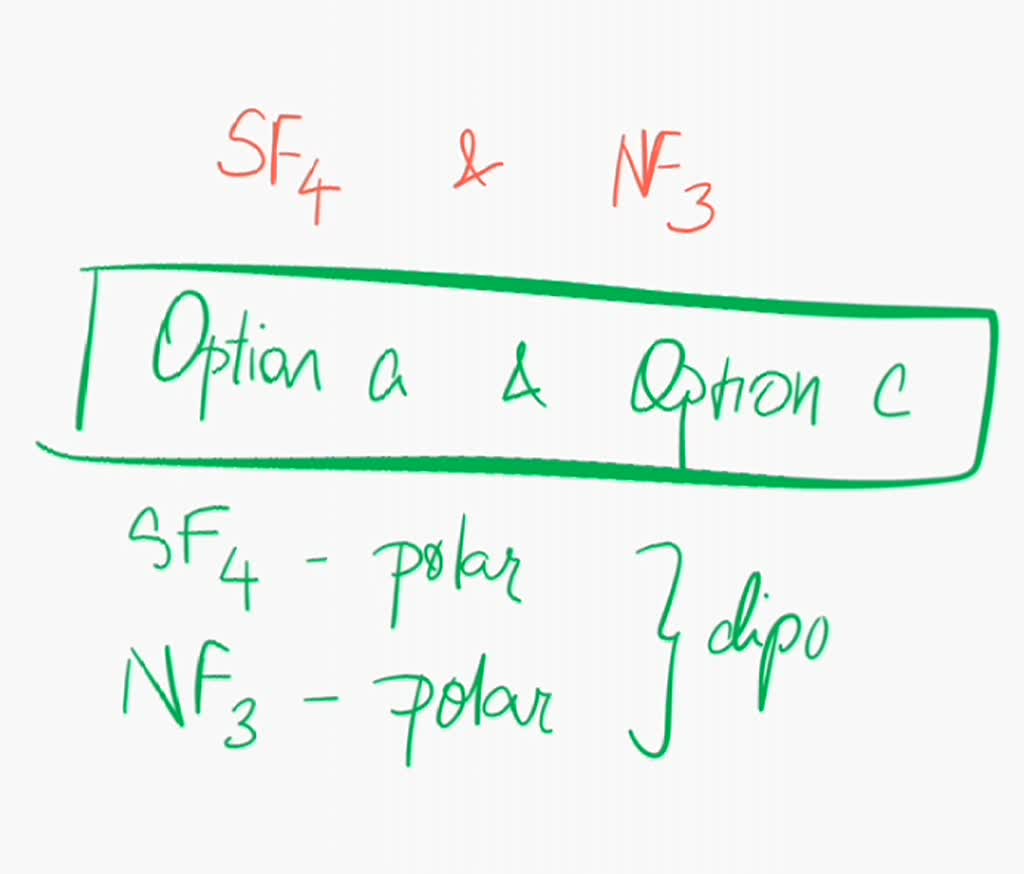
SOLVED Are sulfur tetrafluoride (SF4) and nitrogen trifluoride (NF3
In this article, we will discuss NF3 lewis dot structure, molecular geometry or VSEPR shape, bond angle, hybridization, etc. We will also discuss is NF3 polar or non-polar? NF3 is used to make chemicals or also used as a component of rocket fuels. It is a strong greenhouse gas and it has the potential to reduce global warming greater than co2.

SOLVED Which of the following, if any, contain nonpolar covalent bonds
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Compare and contrast NH3 and NF3. Are they polar or nonpolar compounds? What type of intermolecular force is present for each compound? Which compound has the higher boiling point?
Ppt Polar Bonds And Molecules Powerpoint Presentation, Free Download 587
NF3 is a chemical formula for Nitrogen Trifluoride. It is used in manufacturing semiconductors. Here in this video, we share a detailed step-by-step method t.

BF3 and nf3 both molecules are covalent BF3 is nonpolar and nf3 is
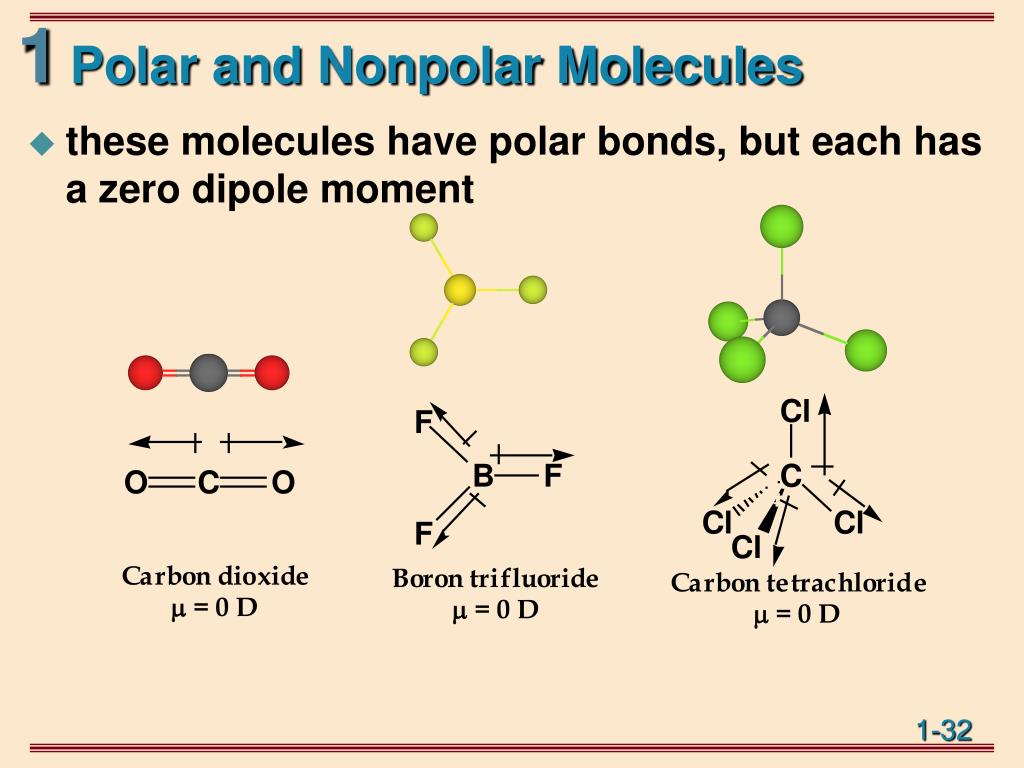
When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning force is given by the formula. µ = q × d. where q is the amount of charge and d is the distance between the two charges. µ is the turning moment.
Solved Classify each molecule as polar or nonpolar. Polar
In this blog post, we will explore the Lewis structure for NF3, its bond angle, and whether it is polar or nonpolar. We will also answer questions like how many bonds NF3 has and dive into its molecular geometry. So, let's dive in and unravel the mysteries behind NF3! Lewis Structure for NF3: A Molecular Dance of Nitrogen and Fluorine
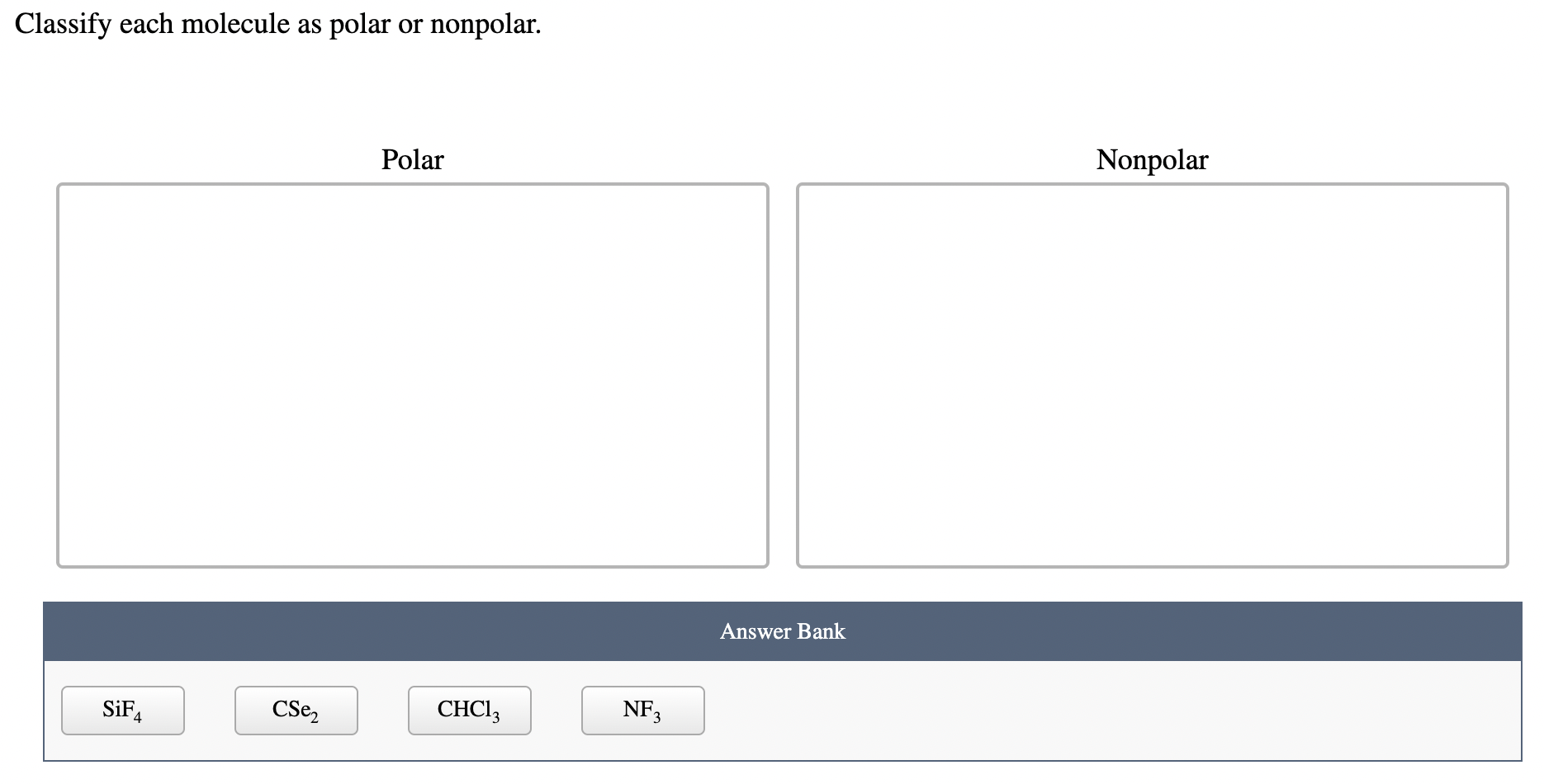
SOLVED Determine whether each molecule given below is polar or
Learn to determine if NF3 (Nitrogen trifluoride) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewi.